Nifedipine sustained-release tablet and preparation method thereof
A technology of nifedipine and nifedipine, applied in the field of medicine, can solve the problems of slow dissolution rate of nifedipine, slow release rate of slow control agent, inability to achieve complete release, etc., and achieve good fluidity and compressibility, good Disintegration effect, anti-triboelectric effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
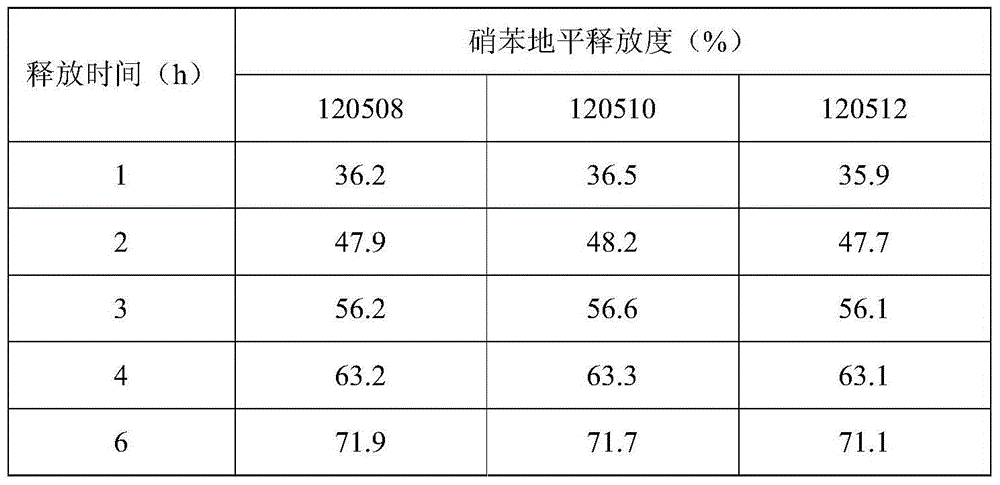
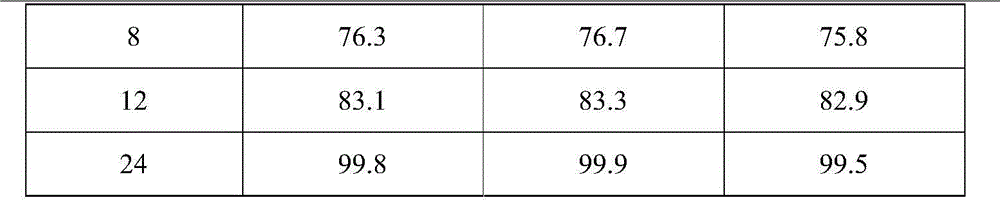
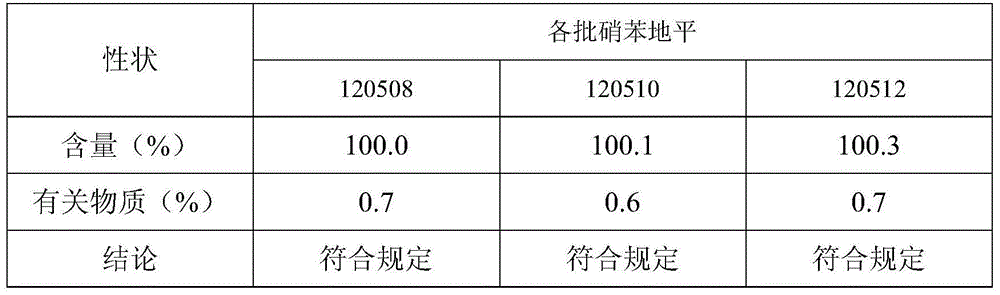
[0030] Based on the nifedipine sustained-release tablets made into 1000 tablets (each containing 20 mg of nifedipine),
[0031] Components and weight of the outer layer tablet: 5g of nifedipine, 10g of microcrystalline cellulose, 30g of spray-dried lactose (FlowLac100), 0.2g of sodium lauryl sulfate, 2.0g of copovidone (Plasdone S-630), micropowder Silica gel 1.0g, magnesium stearate 0.2g;
[0032] The composition and weight of the inner sheet: 15g of nifedipine, 100g of lactose, 45g of pregelatinized starch, 10g of hydroxypropyl cellulose (produced by Taian Ruitai Cellulose Co., Ltd.), 10g of sodium alginate, 10g of potassium alginate, poly Vitamin ketone (K30) 20g, PEG6000 2g, polyvinylpyrrolidone 2.0g, magnesium stearate 1.5g.
[0033] (1) Powder preparation of the outer layer tablet:
[0034] Grind microcrystalline cellulose, spray-dried lactose, copovidone, magnesium stearate and micronized silica gel to pass through an 80-mesh sieve, and set aside; mix nifedipine and s...
Embodiment 2
[0063] Based on the nifedipine sustained-release tablets made into 1000 tablets (each containing 20 mg of nifedipine),
[0064] Components and weight of the outer tablet: 5g of nifedipine, 12g of microcrystalline cellulose, 32g of spray-dried lactose, 0.25g of sodium lauryl sulfate, 2.5g of copovidone, 1.2g of micropowder silica gel, stearic acid Magnesium 0.3g;
[0065] Components and weight of the inner sheet: 15g of nifedipine, 110g of lactose, 50g of pregelatinized starch, 12g of hydroxypropyl cellulose (HPC), 12g of sodium alginate, 12g of potassium alginate, 22g of povidone, PEG6000 2.5g, polyvinylpyrrolidone 2.2g, magnesium stearate 1.8g.
[0066] Preparation method: dissolve nifedipine, povidone and PEG6000 in 600ml of absolute ethanol, and the rest are the same as in Example 1.
Embodiment 3
[0068] Based on the nifedipine sustained-release tablets made into 1000 tablets (each containing 20 mg of nifedipine),
[0069] Components and weight of the outer tablet: 5g of nifedipine, 8g of microcrystalline cellulose, 28g of spray-dried lactose, 0.15g of sodium lauryl sulfate, 1.5g of copovidone, 0.8g of micropowder silica gel, stearic acid Magnesium 0.1g;
[0070] Components and weight of the inner sheet: 15g of nifedipine, 90g of lactose, 40g of pregelatinized starch, 8g of hydroxypropyl cellulose (HPC), 8g of sodium alginate, 8g of potassium alginate, 18g of povidone, PEG6000 1.5g, polyvinylpyrrolidone 1.8g, magnesium stearate 1.2g.
[0071] Preparation method: dissolve nifedipine, povidone and PEG6000 in 400ml of absolute ethanol, and the rest are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com