A kind of preparation method of nonionic ultraviolet light curing waterborne polyurethane acrylate
A polyurethane acrylate, non-ionic technology, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problems of easy degradation and deterioration, short shelf life of non-ionic water-based polyurethane, and achieve environmental friendliness, good storage stability, The effect of expanding the range of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
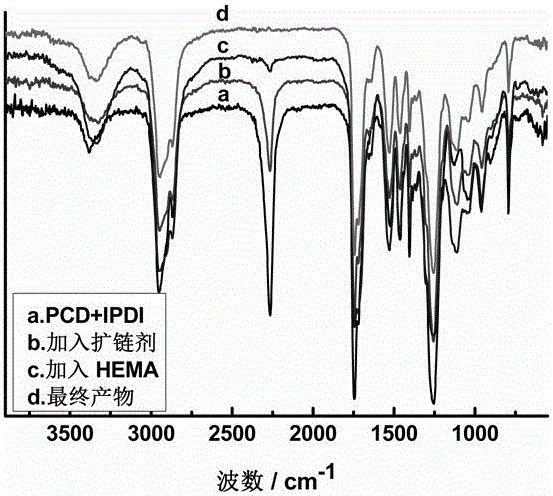
Image
Examples
Embodiment 1
[0033] (1) Synthesis of a chain extender with two hydroxyl groups with a hydrophilic long-chain polymer in the side chain
[0034] Take 11.12 grams (0.05mol) of isophorone diisocyanate into a three-necked flask, and take 50 grams of polyethylene glycol monomethyl ether (molecular weight: 1000, 0.05mol), add 0.018 grams of catalyst dibutyltin dilaurate, in Add dropwise at 45°C, react for 2 hours, until the NCO group content measured by the toluene-di-n-butylamine method reaches the theoretical value of 3.44%, add 5.26 g (0.05mol) of diethanolamine, raise the temperature to 50°C, and continue the reaction for 2 hours , until the NCO characteristic absorption peak disappears and stops when measured by a Fourier transform infrared transform spectrometer;
[0035] (2) Synthesis of non-ionic UV-curable waterborne polyurethane acrylate prepolymer
[0036] Add 100 grams (0.1 mol) of polytetrahydrofuran ether glycol (PTMG) with a number average molecular weight of 1000 and a hydroxyl ...
Embodiment 2
[0040] (1) Synthesis of a chain extender with two hydroxyl groups with a hydrophilic long-chain polymer in the side chain
[0041] As shown in embodiment 1 step (1);
[0042] (2) Synthesis of non-ionic UV-curable waterborne polyurethane acrylate prepolymer
[0043] Add 100 grams (0.1 mol) of polyoxypropylene glycol (PPG) with a number average molecular weight of 1000 and a hydroxyl value of 110 mgKOH / g, and 44.4 grams (0.2 mol) of isophorone diisocyanate (IPDI) into the reaction vessel, React for 3 hours under the action of mechanical stirring at 40°C; after the NCO content measured by the toluene-di-n-butylamine method reaches 8.90%, add 66.3 grams of the chain extender prepared in (1), and heat up to 55°C for 2.5 hours; the NCO content After reaching 2.71%, add 13 grams (0.1mol) of photosensitive monomer hydroxyethyl methacrylate (HEMA) with unsaturated double bonds and raise the temperature to 65°C for 2 hours until the NCO is measured by Fourier transform infrared spectro...
Embodiment 3
[0047] (1) Synthesis of a chain extender with two hydroxyl groups with hydrophilic groups on the side chain
[0048] As shown in embodiment 1 step (1);
[0049] (2) Synthesis of non-ionic UV-curable waterborne polyurethane acrylate prepolymer
[0050] Add 100 grams (0.1 mol) of polycaprolactone diol (PCL) with a number average molecular weight of 1000 and a hydroxyl value of 109-119 mgKOH / g, and 44.4 grams (0.2 mol) of isophorone diisocyanate (IPDI) into the reaction In the container, react under mechanical stirring at 50°C for 1 hour; after the NCO content measured by the toluene-di-n-butylamine method reaches 8.90%, add 66.3 grams of the chain extender prepared in (1), and heat up to 60°C for 2 hours ; After the NCO content reaches 2.63%, add 13 g (0.1 mol) of photosensitive monomer hydroxyethyl methacrylate (HEMA) with unsaturated double bonds and heat up to 70 ° C for 1.5 hours, until the Fourier transform infrared spectrometer It is measured that the characteristic abso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com