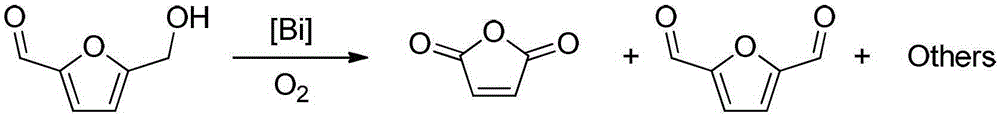
A method for preparing maleic anhydride by catalytic oxidation of 5-hydroxymethylfurfural
A technology for hydroxymethyl furfural and maleic anhydride is applied in the field of a new catalytic system for catalyzing the oxidation of 5-hydroxymethyl furfural by catalyzing molecular oxygen to prepare maleic anhydride, which can solve problems such as limited direct application range, and achieve obvious creativity and novelty. , mild reaction conditions, high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
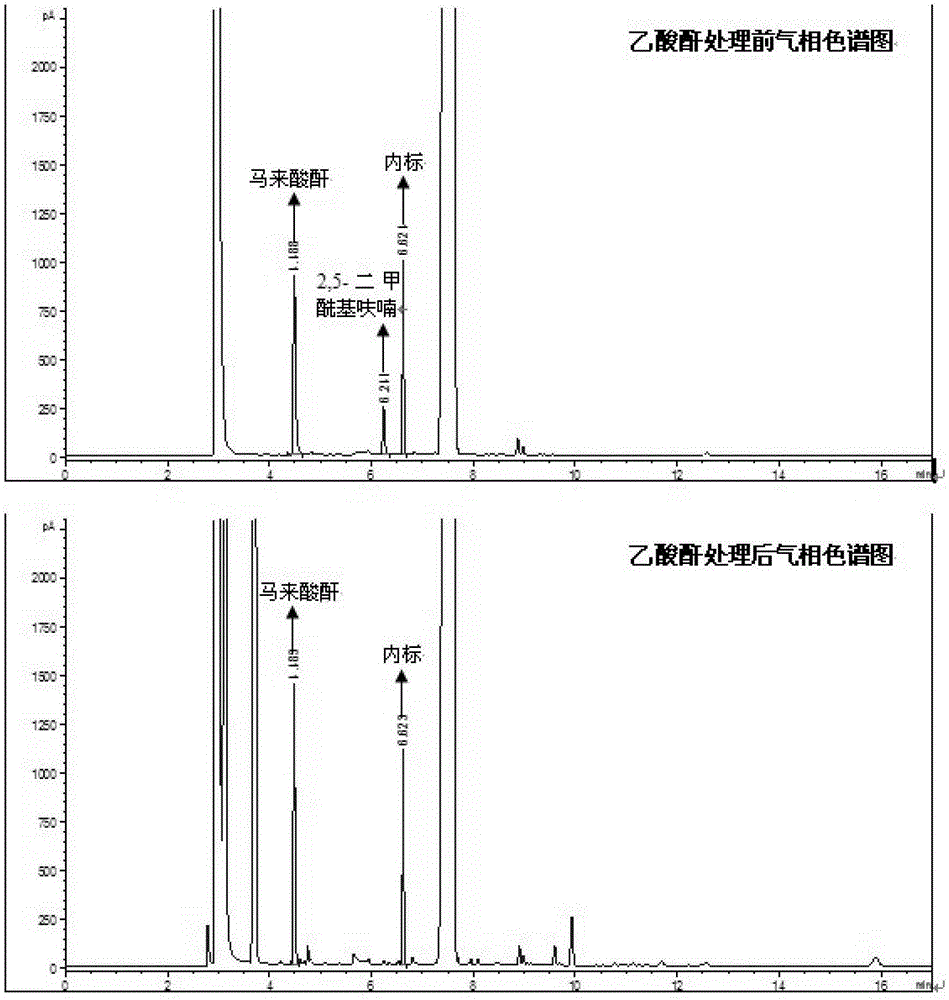
[0019] Add 0.32g of 5-hydroxymethylfurfural and 5mol% (relative to 5-hydroxymethylfurfural) bismuth oxide into a 35mL reaction kettle, add 2mL of sulfolane, close the kettle, fill it with oxygen at a pressure of 1.0MPa, and heat up to 80°C, and keep it for 6h. It was then cooled to room temperature and carefully depressurized to atmospheric pressure. Transfer all the products to a 25mL volumetric flask, add 2mL internal standard (durene) and then make up to volume. The main product was qualitatively analyzed using GC-MS and gas chromatographic retention times of standard substances. Quantitative analysis of the product was carried out by gas chromatography internal standard method. Calculate the yield of maleic anhydride according to the formula yield of maleic anhydride = (maleic anhydride substance amount) / (the amount of substance added with 5-hydroxymethylfurfural before the reaction). Since water is generated during the oxidation reaction, part of the maleic anhydride g...
Embodiment 2
[0021] Add 0.32g of 5-hydroxymethylfurfural and 2mol% (relative to 5-hydroxymethylfurfural) bismuth nitrate into a 35mL reaction kettle, add 2mL of dimethyl sulfoxide, close the kettle, and fill it with oxygen at a pressure of 0.5MPa. The temperature was raised to 60°C under stirring, and kept for 8h. It was then cooled to room temperature and carefully depressurized to atmospheric pressure. After the treatment with acetic anhydride, transfer all the products to a 25mL volumetric flask, add 2mL of internal standard and then make up to volume. The product was quantitatively analyzed according to the method in Example 1. Calculate the total yield of maleic anhydride to be 38.7%.
Embodiment 3-15
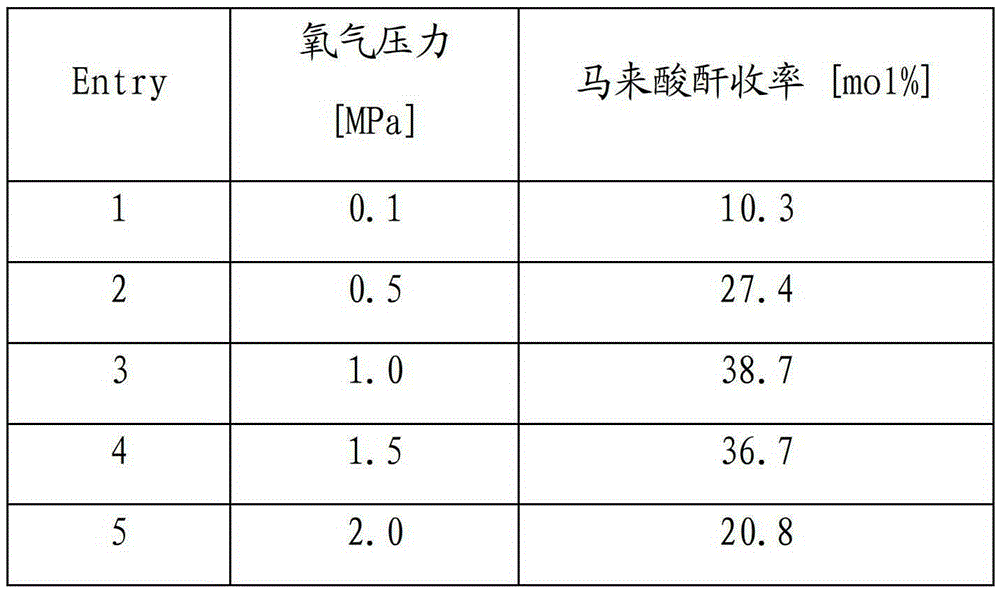
[0023] Bismuth oxide, bismuth hydroxide, bismuth fluoride, bismuth chloride, bismuth bromide, bismuth nitrate, bismuth sulfate, bismuth phosphate, bismuth subcarbonate, bismuth acetate, bismuth ferrite, bismuth titanate, bismuth vanadate, etc. catalytic activity. Reaction conditions: 0.32g 5-hydroxymethylfurfural, 2.5mol% catalyst (relative to 5-hydroxymethylfurfural), 2mL 1,4-dioxane, 1.0MPa oxygen, 100°C, 4h, after the reaction, cool to room temperature, carefully depressurized to atmospheric pressure. After the treatment with acetic anhydride, transfer all the products to a 25mL volumetric flask, add 2mL of internal standard and then make up to volume. Quantitative analysis of product according to the method in embodiment 1, obtains the total yield of maleic anhydride as shown in table 1.
[0024] Table 1 The influence of different bismuth-based catalysts on the yield of maleic anhydride under the same reaction conditions
[0025] Entry
[0026] Conclusion: Bis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com