Protein nanoparticle containing bioactivity oligopeptide-ferritin heavy chain subunit and preparation method of protein nanoparticle
A ferritin heavy chain and biologically active technology, which is applied in the field of protein nanoparticles containing bioactive short peptide-ferritin heavy chain subunits and its preparation, can solve problems such as incorrect folding and assembly, and achieve the goal of avoiding interactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
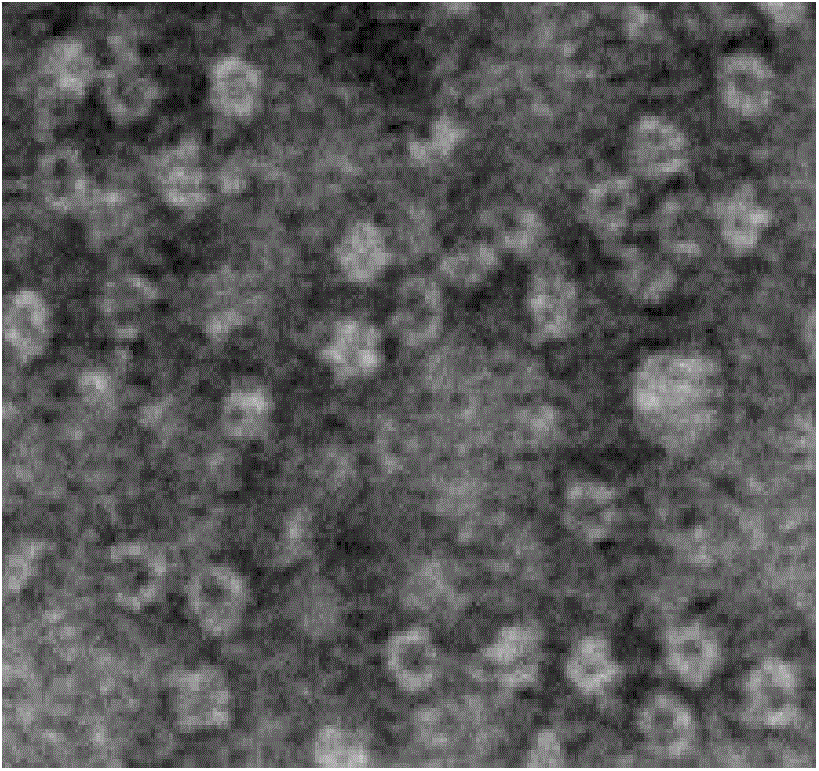
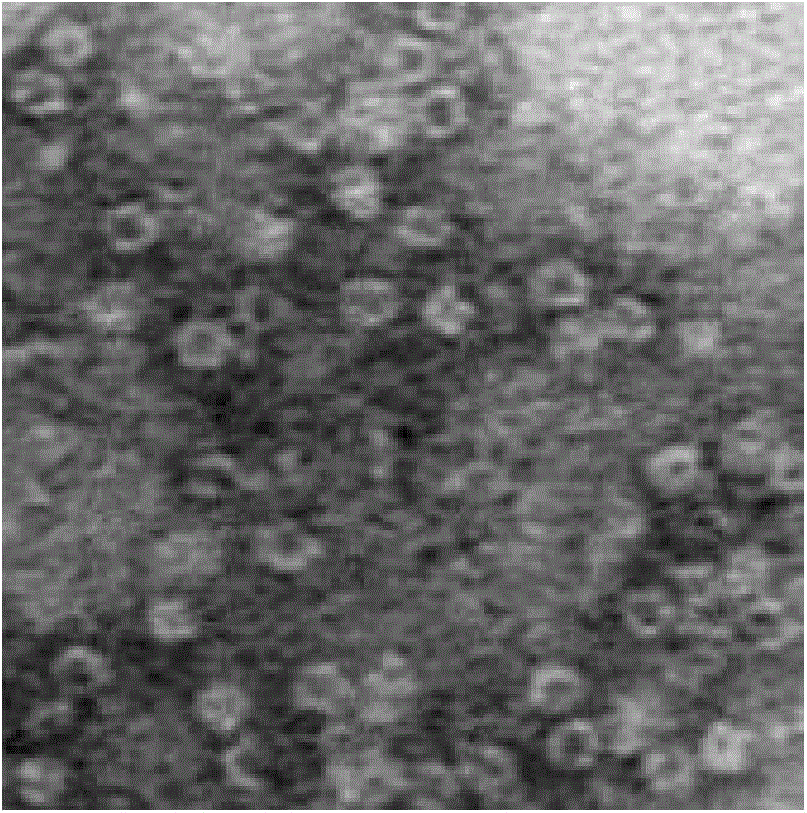
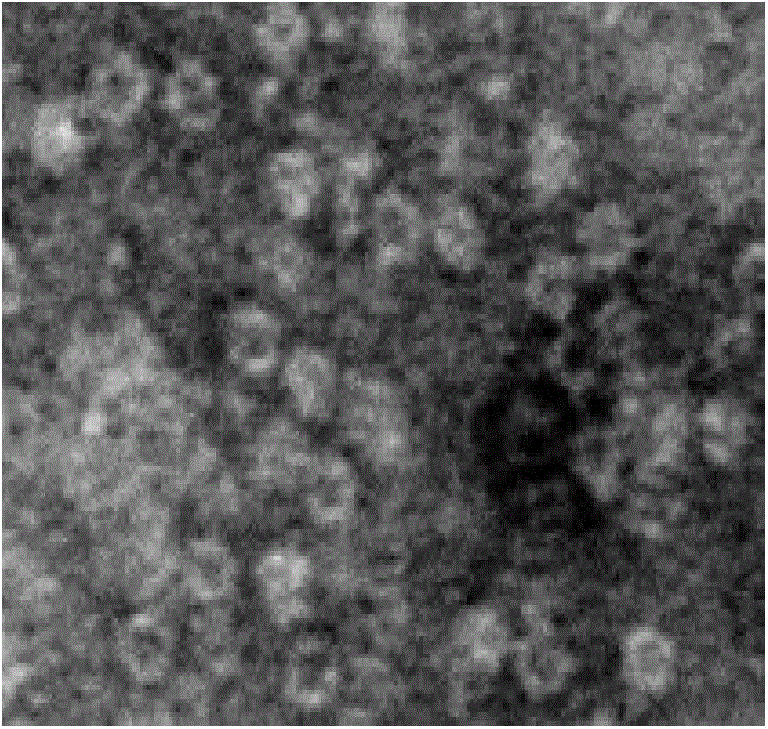
Image
Examples
Embodiment 1
[0036] Example 1 Construction of pET28a(+) / HSA-1-FTH1 expression engineering bacteria:
[0037] According to the sequence of the target gene, a pair of forward and reverse primers were designed and named as HSA-1-F1 and HSA-1-R1, HSA-1-F2 and HSA-1-R2. Design appropriate protective bases and restriction sites on both sides of the target sequence, the restriction sites are EcoR I and Nco I. Firstly, HSA-1-F2 and HSA-1-R2 were used as primers and templates to carry out the first round of PCR to obtain corresponding products. Then use HSA-1-F1 and HSA-1-R1 as PCR primers, and use the product of the first round as a template to carry out the second round of PCR to obtain the target gene fragment HSA-1. Subsequently, the target gene fragment was double digested and inserted between the restriction sites EcoR I and Nco I of the pET-28(+)-FTH1 plasmid to obtain the target recombinant plasmid.
[0038] The following is the primer sequence of HSA-1, the sequence is in the 5'-3' direc...
Embodiment 2
[0052] The preparation of embodiment 2HSA-1-FTH1 target protein
[0053] Induction of the obtained Pet28a(+) / HSA-1: FTH1 expression bacteria prepared in Example 1 to express HSA-1: FTH1 target protein: pick the monoclonal engineering expression colony from the plate, and put it in 25ml LB liquid medium (40% NaCI, 40% Tryptone, 20% Yeast Extract, kanamycin 50 μg / ml), placed in a constant temperature shaker at 37° C., 200 rpm, and cultured overnight. On the next day, inoculate 1% of the inoculum into 200ml LB liquid medium (the composition and antibiotic concentration are the same as above). As for the 37°C constant temperature shaker, when the OD is about 0.6, add a final concentration of 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG), and induce overnight at 37°C. Finally, the bacterial cells were collected by centrifugation at 4°C and 5000 rpm for 30 minutes, and the bacterial cells were stored at -20°C.
Embodiment 3
[0054] The preparation of embodiment 3HSA-3-FTH1 target protein
[0055] The pET28a(+) / HSA-3-FTH1 expressing engineered strain was constructed and induced to express HSA-3-FTH1 protein. The PCR reaction steps and the establishment and expression methods of the expressing engineered strain were as described in Examples 1 and 2.
[0056] The following is the primer sequence of HSA-3, the sequence is in the 5'-3' direction:
[0057] HSA-3-F1 (SEQ ID NO: 13):
[0058] TCATGCCATGGCAAAGGAACAGCTAAAGGCAGTAATGGACGACTTCGCAGCAT;
[0059] HSA-3-R1 (SEO ID NO: 14):
[0060] ACCGGAATTCACTTCCTCCTCCTCCACTT CCTCCTCCTCCGTCGACACAC;
[0061] HSA-3-F2 (SEQ ID NO: 15):
[0062] CAGTAATGGACGACTTCGCAGCATTCGTAGAAAAGTGTGTC;
[0063] HSA-3-R2 (SEQ ID NO: 16):
[0064] TCCTCCTCCTCCGTCGACACACTTTTTCTACGAATGCTGC.
[0065] The amino acid sequence of the HAS-3 active short peptide is shown in SEQ ID NO: 2 in the sequence listing, and the amino acid sequence of the obtained HSA-3-FTH1 target fusion prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com