Quick detection method and kit for trace-amount endotoxin
A technology of detection kits and detection methods, which are applied in measurement devices, preparation of test samples, instruments, etc., can solve the problems of killing a large number of protected animals, the color and turbidity of the test result samples themselves, and improving the detection The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Sensitivity analysis of MIP-ELISA method
[0037] Add a series of concentration gradient endotoxin standards (10, 50, 100, 1000, 10000, 100000ng / mL) into the wells of the enzyme-labeled plate containing artificial antibodies, 100μL per well, 20-40℃ after 1-5h Wash 2-8 times with phosphate buffer. Then add 100μL of rabbit anti-E. coli endotoxin polyclonal antibody to each well (diluted with antibody diluent at 1:100~1:2000), incubate at 20-40℃ for 1-5h and wash 2-8 times with phosphate buffer . Add 100μL of horseradish peroxidase-labeled goat anti-rabbit antibody to each well (diluted with antibody diluent at 1:500~1:5000), incubate at 20-40℃ for 1-5h and wash with phosphate buffer 2 -8 times. Finally, add 200μL of enhanced luminescence working solution to each well (molar concentration is 5*10 -4 mol / L Luminol, 4*10 -3 mol / L of H 2 O 2 And 4*10 -4 mol / L PIP), immediately use the multifunctional microplate reader to detect the luminous intensity.
[0038] From ...
Embodiment 2
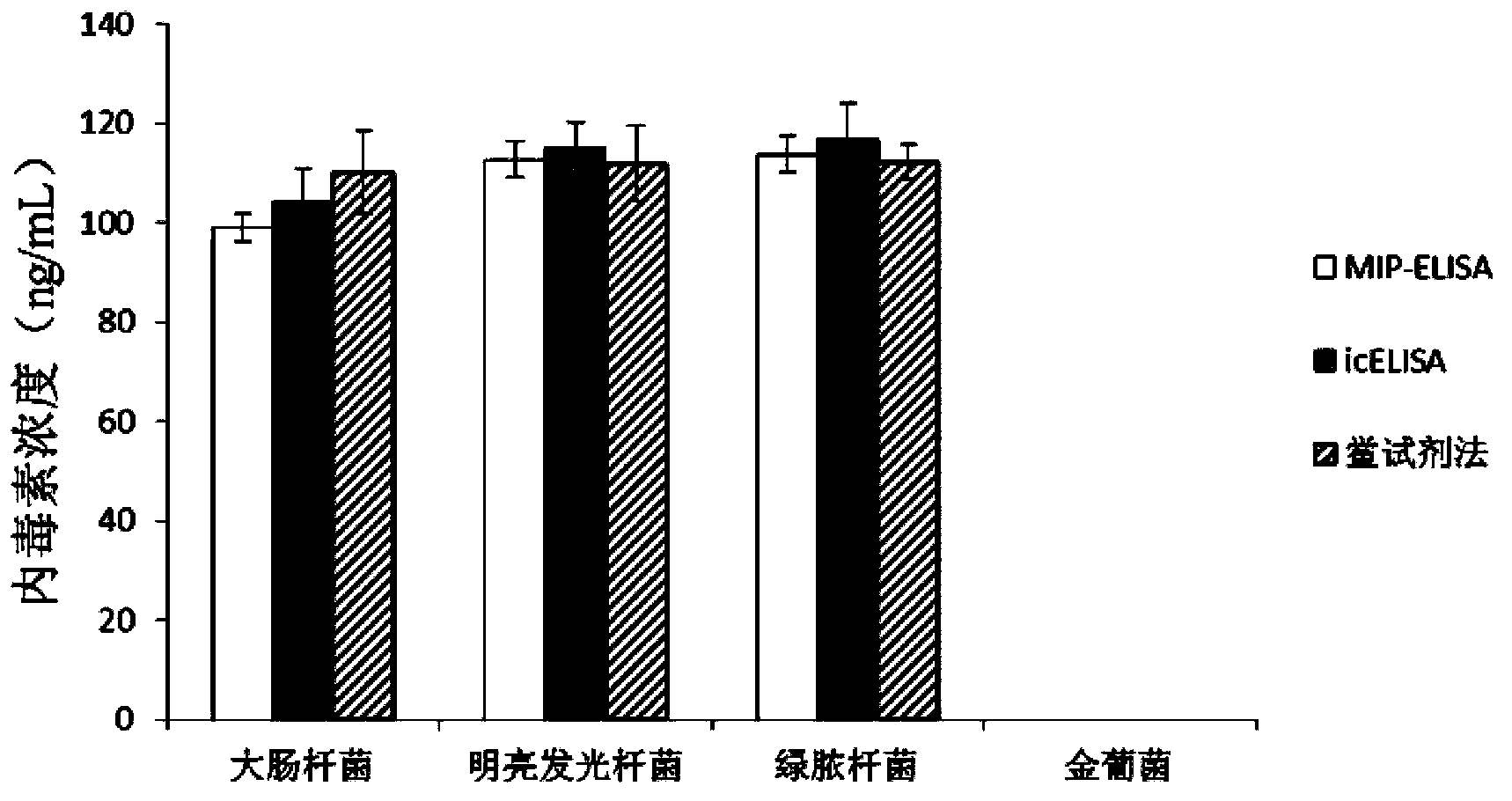
[0039] Example 2: Specificity analysis of MIP-ELISA
[0040] The MIP-ELISA method was used to determine the endotoxin content produced by various bacteria, and compared with the standard method limulus reagent method and icELISA method. Cultivate 3 kinds of Gram-negative bacteria (E. coli, Photobacterium luminescens, Pseudomonas aeruginosa), and 1 kind of Gram-positive bacteria (Staphylococcus aureus). Combine 4 kinds of bacteria liquid (all 10 5 cfu / mL) boiled in boiling water for 2.5h to inactivate, inactivate the endotoxin in the bacteria and release it. The endotoxin content in various bacterial lysates was determined according to the MIP-ELISA method established in Example 1. The endotoxin concentrations in Gram-negative bacteria-Escherichia coli, Photobacterium luminescens and Pseudomonas aeruginosa were 99.15±2.79 ng / mL, 112.63±3.68ng / mL, 113.74±3.58ng / mL, the test result of Gram-positive bacteria-Staphylococcus aureus was negative. Simultaneously use the standard limulu...
Embodiment 3
[0041] Example 3: MIP-ELISA method and stability analysis of the corresponding kit
[0042] (1) Method stability. Place the collected river water (Wuhan Yangtze River) in a container and let it settle naturally for 24 hours to remove large particles and suspended solids. Different concentrations of endotoxin standards (10, 50, 100 ng / mL) are added to the post-settlement In the river water samples, the endotoxin content in the unspiked and spiked river water samples was determined according to the MIP-ELISA method established in Example 1. The recovery rate of endotoxin addition was 83.6%-101.7%, and the intra-day deviation was 2.1~ 4.0%, the day-to-day deviation is 1.6-4.6%, all less than 5% (n=6). At the same time, icELISA was used to detect the same sample. The recovery rate of endotoxin was 79.3%-92.5%, the intra-day deviation was 4.2-7.1%, and the inter-day deviation was 3.9-8.6% (n=6). It shows that MIP-ELISA has good stability, while the icELISA which uses natural antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com