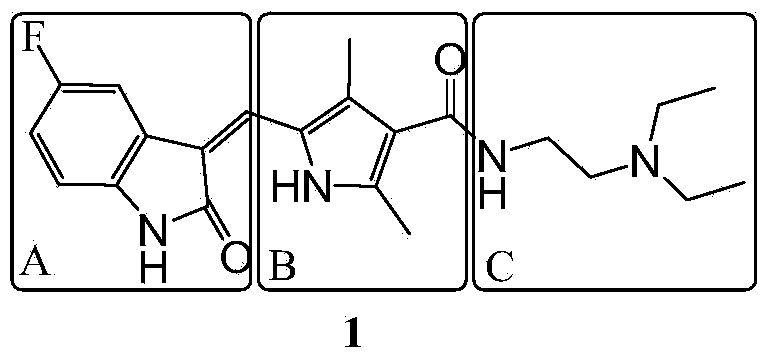
Method for preparing sunitinib
A technology of sunitinib and dimethyl, which is applied in the field of preparation of sunitinib, can solve the problems of long production cycle, high production cost and low total yield, achieve long reaction time, reduce synthesis cost, and synthesize The effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
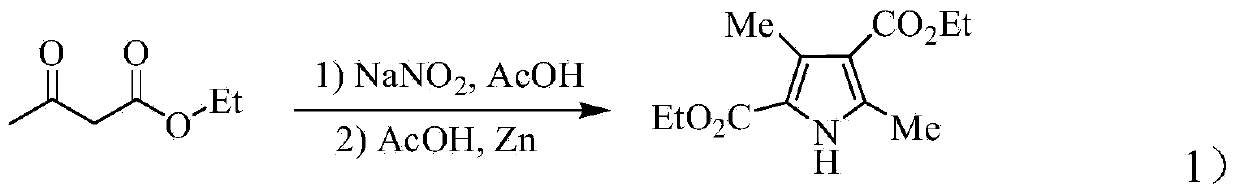
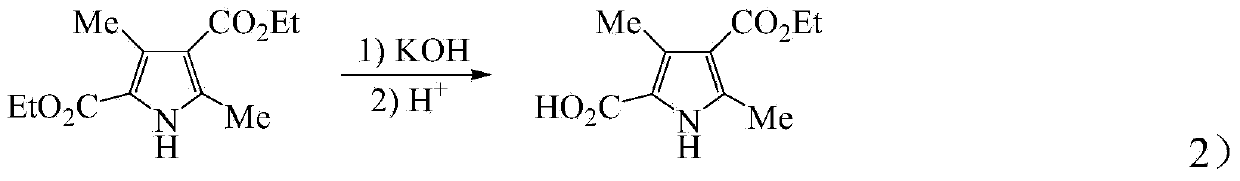
[0044] The improvement steps in the preparation method of sunitinib of the present invention are as follows: 1) Pre-increasing the temperature of 3,5-dimethyl-1H-pyrrole-2,4-bis by controlling the temperature when adding sodium nitrite and zinc powder The productive rate of diethyl carboxylate; 2) to find out synthetic 3,5-dimethyl-1H-pyrrole-4-ethoxycarbonyl-2-carboxylate by the investigation of factors such as feed ratio, alkali concentration, reaction temperature; 3) propose a new synthetic method for 2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester); 4) improve N-(2-diethylaminoethyl)-2 , 4-dimethyl-5-formyl-1H-pyrrole-3-carboxamide post-treatment method to more simply remove the by-product dicyclohexyl urea (DCU), and improve the yield of compound 8; 5) Shorten the synthesis steps of 5-fluoro-1,3-indolin-2-one; 6) improve the optimal reaction conditions for the synthesis of sunitinib and increase its yield through the selection of solvents and catalysts.
[0045] (1...
Embodiment 1
[0053] Add 47.2mmol of ethyl acetoacetate and 12mL of glacial acetic acid into a 100mL three-necked bottle equipped with a thermometer and a magnet, and add dropwise nitrous acid with a concentration of 23.6mmol of sodium nitrite and a concentration of 9.8mol / L under stirring in an ice bath. Sodium aqueous solution, and the temperature is controlled at 0°C during the dropwise addition process. After the addition, react in an ice bath for 2.5 hours, then raise the temperature to 30°C and add 47.2mmol of zinc powder in batches. After the addition, remove the ice bath and replace it with constant temperature heating Magnetic stirrer, heated to reflux for 1h, the reaction system was a yellow liquid at this time. The reaction solution was poured into 80 mL of ice water while it was hot, and a light yellow solid precipitated out, which was filtered with suction, and the filter cake was washed with ice water. Dry to obtain 5.33g of 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate diethyl es...
Embodiment 2
[0056]Add 236.0mmol of ethyl acetoacetate and 60mL of glacial acetic acid into a 25mL three-neck bottle equipped with a thermometer and a magnet, and add dropwise under stirring in an ice bath. L of sodium nitrite aqueous solution, and the temperature was controlled at 5°C during the dropping process. After the addition was completed, it was reacted under ice bath conditions for 2.5h, then the temperature was raised to 25°C and 236.0mmol zinc powder was added in batches at this temperature, and the After completion of the reflux reaction for 1.0h, the reaction system was a yellow liquid. Pour the reaction solution into 400mL of ice water while it was hot, and a large amount of yellow solid precipitated out, then stood still for 2-3h, filtered with suction, washed the filter cake with ice water and dried, and weighed to obtain 26.7g of yellow crystal 3,5-dimethyl-1H -Diethyl pyrrole-2,4-dicarboxylate, the crude yield is 94.4%. m.p.133-134°C (lit.135-136°C; Schnettler RA, Dage ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com