Identification method for fingerprint spectrum of salvia miltiorrhiza medicinal material
A technology of fingerprint and Salvia miltiorrhiza, applied in the identification field of Salvia miltiorrhiza medicinal materials, can solve the problems of few components, poor reproducibility of the same sample, few chemical components, etc., and achieve a good separation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
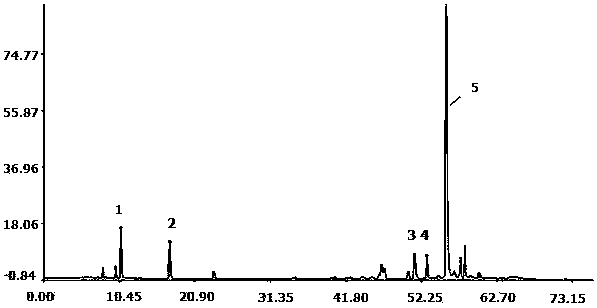
[0033] Example 1: Determination of Fingerprint Spectrum of Salvia Miltiorrhiza
[0034]1. Instruments: Agilent 1260 high performance liquid chromatography, G1311C quaternary pump, column thermostat, G1316A, G1315D, DAD detector, Agilent Zorbax Eclipse XDB C18 column (4.6×250mm, 5μm). The mass spectrometer system is an ESI-MSn mass spectrometer (Bruker).
[0035] [0025 Reagents: Acetonitrile was chromatographically pure (Burdick&Jackson, Honeywell International Inc., USA), glacial acetic acid was chromatographically pure (Tedia company Inc., USA), water (Millipore, Bedford, MA, USA), and other reagents were analytically pure (Sinopharm Group Chemical Reagents Ltd). Danshensu, protocatechualdehyde and salvianolic acid B reference substances were purchased from China Biological Products Co., Ltd. Salvianolic acid A, salvianolic acid E, and salvianolic acid D are self-made in this laboratory. All samples are reserved in the Sample Room of the Modernization Center of Traditi...
Embodiment 2
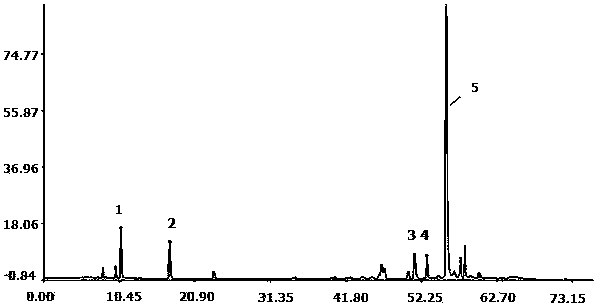
[0059] Embodiment 2: Determination of Fingerprint Spectrum of Salvia Miltiorrhiza
[0060] 1. Instruments: Agilent 1260 high performance liquid chromatography, G1311C quaternary pump, column thermostat, G1316A, G1315D, DAD detector, Agilent Zorbax Eclipse XDB C18 column (4.6×250mm, 5μm). The mass spectrometer system is an ESI-MSn mass spectrometer (Bruker).
[0061] Reagents: Acetonitrile was chromatographically pure (Burdick&Jackson, Honeywell International Inc., USA), glacial acetic acid was chromatographically pure (Tedia company Inc., USA), water (Millipore, Bedford, MA, USA), and other reagents were analytically pure (Sinopharm Group Chemical Reagents Ltd). Danshensu, protocatechualdehyde and salvianolic acid B reference substances were purchased from China Biological Products Co., Ltd. Salvianolic acid A, salvianolic acid E, and salvianolic acid D are self-made in this laboratory. All samples are reserved in the Sample Room of the Modernization Center of Traditional...
Embodiment 3
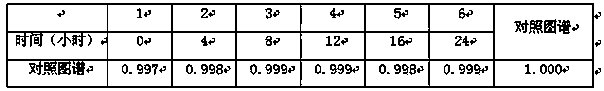
[0071] Embodiment 3: Determination of Fingerprint Spectrum of Salvia Miltiorrhiza
[0072] 1. Instruments: Agilent 1260 high performance liquid chromatography, G1311C quaternary pump, column thermostat, G1316A, G1315D, DAD detector, Agilent Zorbax Eclipse XDB C18 column (4.6×250mm, 5μm). The mass spectrometer system is an ESI-MSn mass spectrometer (Bruker).
[0073] Reagents: Acetonitrile was chromatographically pure (Burdick&Jackson, Honeywell International Inc., USA), glacial acetic acid was chromatographically pure (Tedia company Inc., USA), water (Millipore, Bedford, MA, USA), and other reagents were analytically pure (Sinopharm Group Chemical Reagents Ltd). Danshensu, protocatechualdehyde and salvianolic acid B reference substances were purchased from China Biological Products Co., Ltd. Salvianolic acid A, salvianolic acid E, and salvianolic acid D are self-made in this laboratory. All samples are reserved in the Sample Room of the Modernization Center of Traditional...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com