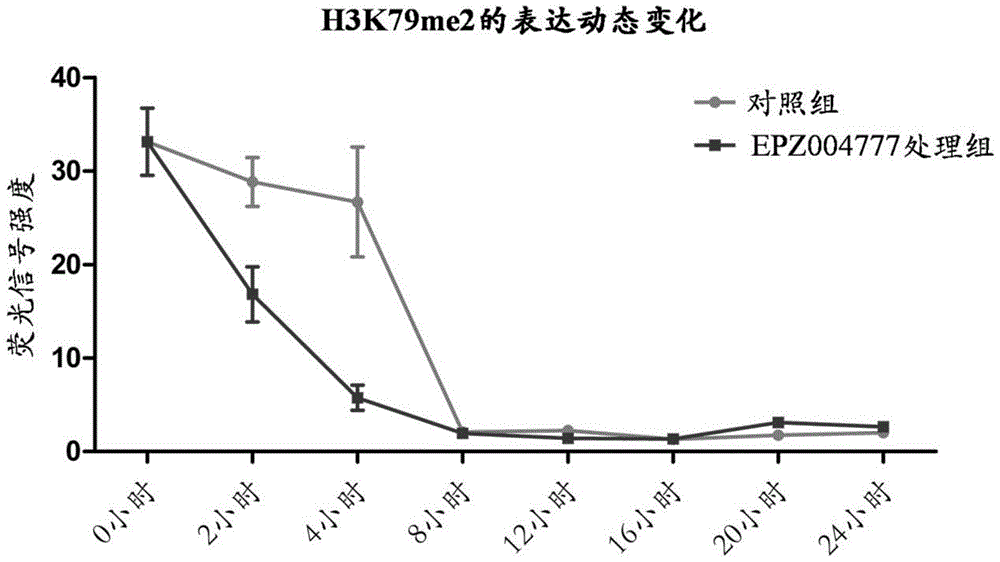
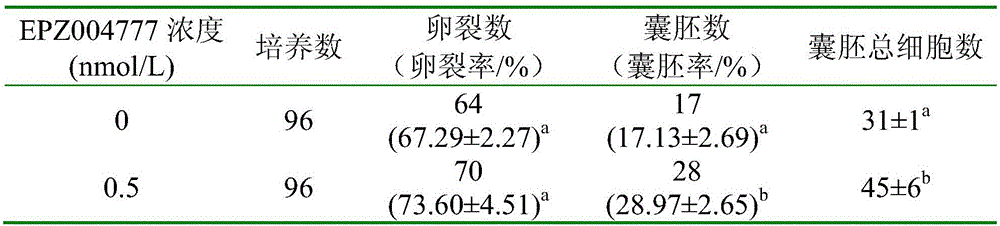
A culture medium for promoting in vitro development of porcine somatic cell cloned embryos
A technology for cloning embryos and in vitro development, applied to artificial cell constructs, animal cells, vertebrate cells, etc., can solve the problems of incompleteness, slow embryonic state, low cloning efficiency, etc., and achieve short half-life and high biological safety , the effect of increasing the quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1. Preparation of porcine oocyte maturation culture medium
[0024] Add 10% porcine follicular fluid, 5% fetal bovine serum, 10IU / mL pregnant horse serum gonadotropin, 10IU / mL human chorionic gonadotropin, 10ng / mL epidermal growth factor, Prepared with 100IU / mL penicillin and 100μg / mL streptomycin.
Embodiment 2
[0025] Example 2. Acquisition and in vitro maturation of pig oocytes
[0026] Put the pig ovary just removed from the slaughterhouse into 37°C normal saline containing penicillin and streptomycin, and transport it back to the laboratory within 2 hours; the retrieved ovary is sprayed with 75% alcohol for disinfection once, and sterile normal saline Wash 3 times, and then use a 10mL disposable plastic syringe equipped with a 18-gauge needle to extract follicles with a diameter of 2 to 6mm on the ovary, and inject the extracted liquid into a 15mL centrifuge tube placed in a constant temperature water bath at 38.5°C. Place the centrifuge tube on a constant temperature heating platform at 38.5°C for 15 minutes to allow the cumulus-oocyte complexes (COCs) to settle naturally; discard the supernatant in the centrifuge tube and add egg washing solution (DPBS buffer containing 0.01% PVA) to dilute and mix the precipitate, and quickly pick up COCs with uniform cytoplasm, more than two l...
Embodiment 3
[0028] Example 3. Obtaining porcine MII stage oocytes and preparing donor cells for nuclear transfer
[0029] Transfer the mature COCs after 42±2 hours into the DPBS buffer containing 1 mg / mL Hya hyaluronidase that has been preheated at 38.5°C, and then gently and repeatedly pipette 200 times or about 3 minutes with a pipette. Afterwards, check under the microscope whether the cumulus cells around the COCs have been completely removed, transfer the oocytes that have been removed from the cumulus into T2 operating solution (TCM199 containing 2% fetal bovine serum), wash 3 times, and transfer to mineral oil to cover In the T2 drops; select oocytes with obvious perivitelline space, complete egg cell membrane, and discharge of the first polar body, which are MII stage oocytes.
[0030] The nucleus donor cells are pig fetal fibroblasts or ear fibroblast somatic cells that have been cultured to the 3rd to 9th passages, and the cells with a confluence of 70% to 90% are selected. One ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com