Compound preparation containing capecitabine for treating gastric cancer
A technology for gastric cancer and pharmaceutical preparations, which is applied in the field of compound preparations containing capecitabine for the treatment of gastric cancer, can solve problems such as irritation, gastrointestinal bleeding and bone marrow suppression, and achieves reduction of treatment dosage, production cost and strong wear resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
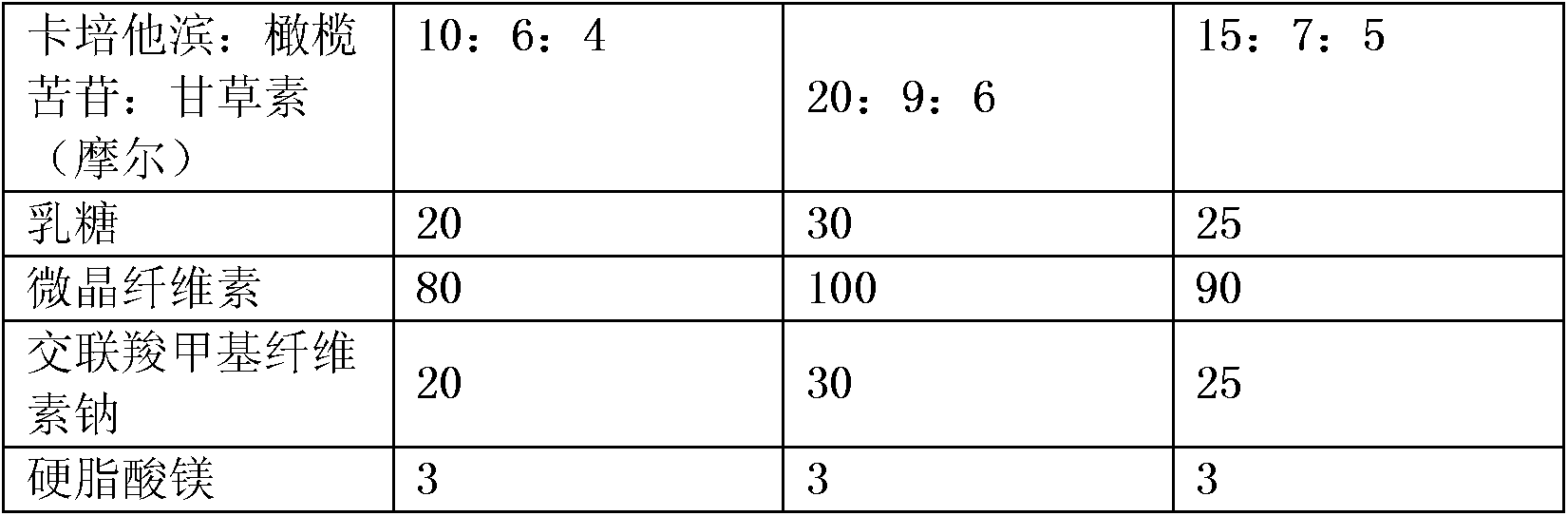
[0031] The molar ratio of the three compounds in the pharmaceutical composition, and the weight ratio (in single tablet) of related auxiliary materials are shown in the table below:
[0032]
[0033]
[0034] Preparation Process:
[0035] (1) Weighing the pharmaceutical composition, lactose, microcrystalline cellulose and croscarmellose sodium and mixing;
[0036] (2) Put the mixed material in a wet granulator and stir for 3 to 5 minutes. After the stirring is completed, add 10% ethanol aqueous solution to the material to make wet granules. Granulator wet granulation;
[0037] (3) Drying: Put the wet granules into the drying equipment, the air inlet temperature is not higher than 80°C, and the moisture content is controlled to be less than 2%;
[0038] (4) Grain sizing: dry sizing with a sizing machine;
[0039] (5) Total blending: add magnesium stearate to the granulated granules, mix; compress into tablets to obtain.
Embodiment 4
[0040] The experimental research of embodiment 4 pharmaceutical composition of the present invention
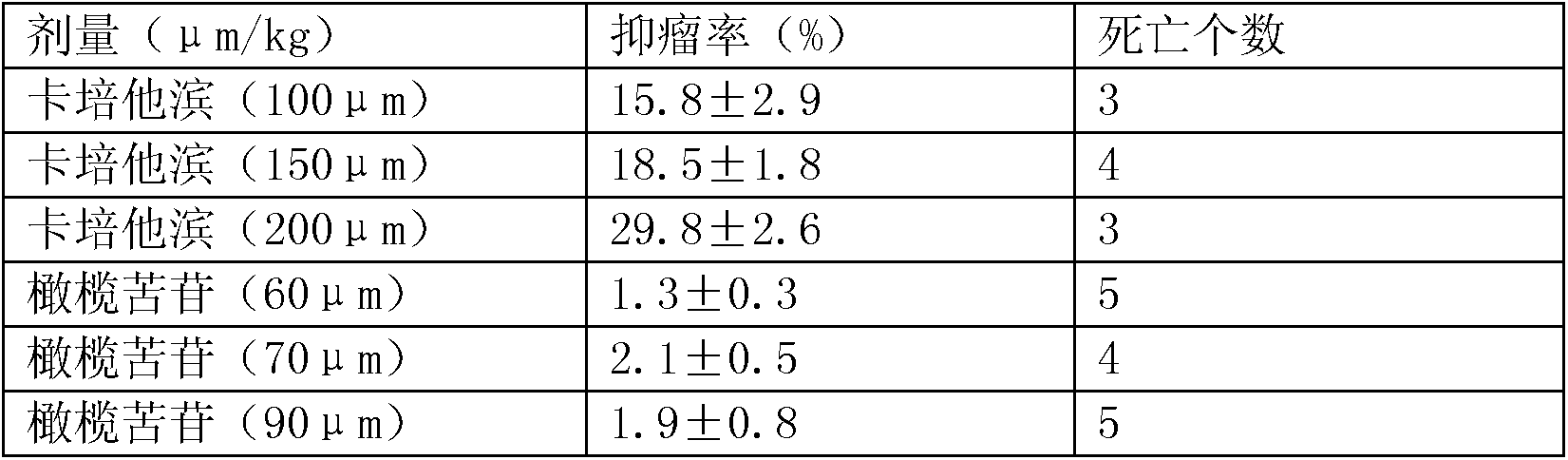
[0041] Human gastric cancer cell BGC-823 was cultured in RPMI1640 culture solution (penicillin 100 U / ml, streptomycin 100 mg / L) with volume fraction of 10% inactivated fetal bovine serum, at 37°C, 5% CO 2 , cultured under saturated humidity conditions. Collect the cells in the logarithmic growth phase, stain with 0.4% trypan blue, detect that the cell viability is higher than 95%, and adjust the concentration to 1.0×10 7 ml of cell suspension. Under sterile conditions, the cell suspension was inoculated subcutaneously in the right armpit of nude mice at 0.2 ml / only, and kept for 2 weeks to establish a tumor-bearing nude mouse model.
[0042] The animals were fed with drinking water and oral medicines respectively. 15 animals in each group were administered once a day for 7 consecutive days. After the last administration, the animals were killed by dislocation of the cervic...
Embodiment 5
[0046] The experimental research of embodiment 5 pharmaceutical composition of the present invention
[0047]Human gastric cancer cell line SGC-7901 was cultured in RPMI1640 medium with volume fraction of 10% inactivated fetal bovine serum (penicillin 100U / ml, streptomycin 100mg / L), at 37°C, 5% CO 2 , cultured under saturated humidity conditions. Collect the cells in the logarithmic growth phase, stain with 0.4% trypan blue, detect that the cell viability is higher than 95%, and adjust the concentration to 1.0×10 7 ml of cell suspension. Under sterile conditions, the cell suspension was inoculated subcutaneously in the right armpit of nude mice at 0.2 ml / only, and kept for 2 weeks to establish a tumor-bearing nude mouse model.
[0048] The animals were fed with drinking water and oral medicines respectively. 15 animals in each group were administered once a day for 7 consecutive days. After the last administration, the animals were killed by dislocation of the cervical spin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com