Method for producing imide salt
A technology of imide salt and thionyl chloride, applied in chemical instruments and methods, iminodisulfonic acid/nitrilotrisulfonic acid, nitrosyl chloride, etc., can solve problems such as time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
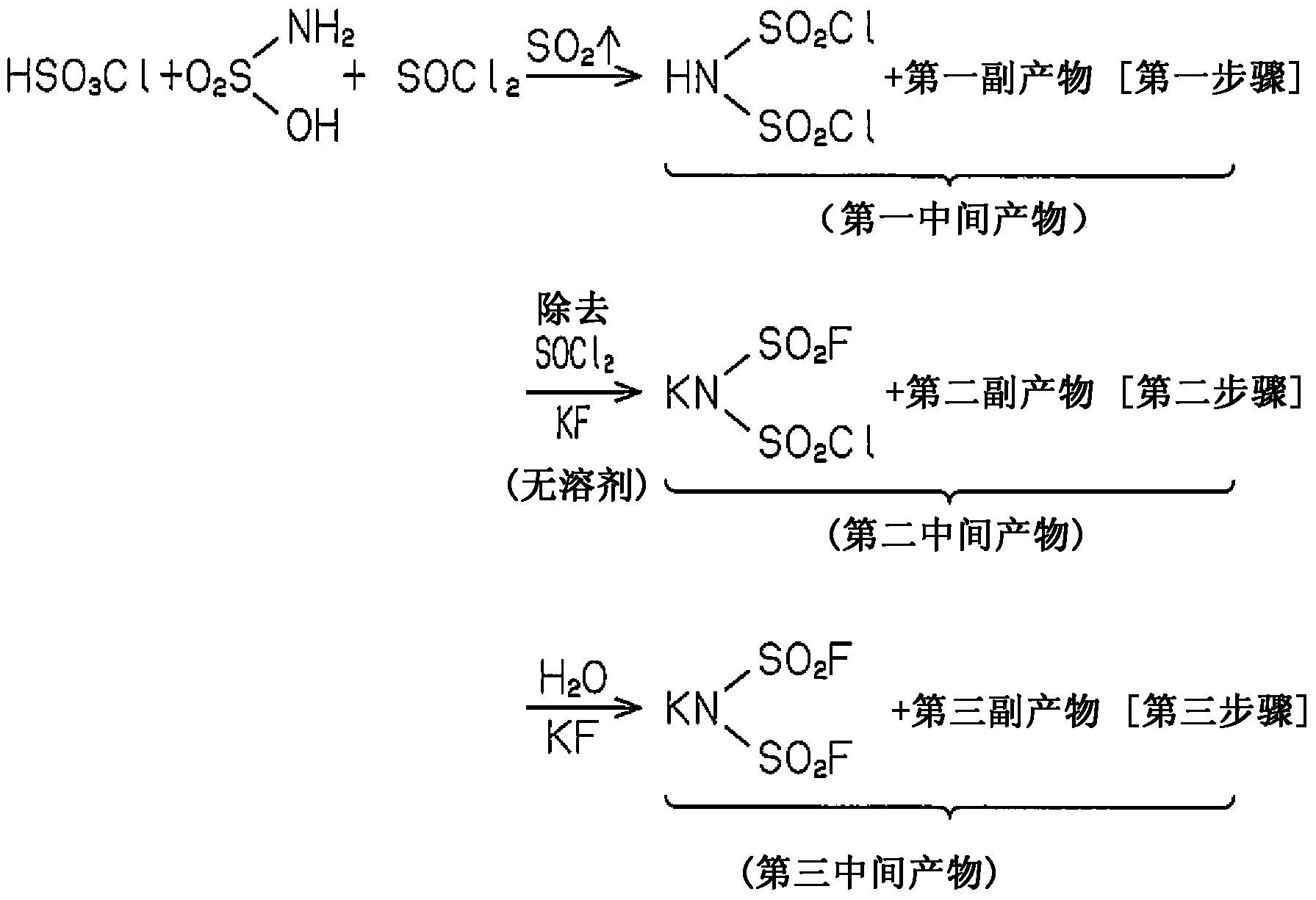
[0036] (first step)
[0037] Sulfamic acid, chlorosulfonic acid, and thionyl chloride were mixed at a molar ratio of 1.0:1.0:2.4 in an inert atmosphere, and heated under reflux. The temperature was 130°C.
[0038] The reaction of sulfamic acid with chlorosulfonic acid and thionyl chloride gives HN(SO 2 Cl) 2 . The reaction also produces by-products including sulfur dioxide, sulfuric acid and hydrochloric acid. Sulfur dioxide is produced from the reaction of thionyl chloride with sulfamic acid. Therefore, the production of sulfur dioxide indicates the continuation of the reaction. Therefore, the above heating is continued until the formation of sulfur dioxide ceases.
[0039] After the reaction was completed, a calcium chloride tube was carefully attached to the vapor outlet of the reaction system so that moisture was not introduced into the reaction system, and the entire reaction system was cooled. Thus, preventing HN(SO 2 Cl) 2 of hydrolysis.
[0040] (second step)...
Embodiment 2
[0052] describes the manufacture of KN(SO 2 F) 2 Methods.
[0053] In Example 2, fluorosulfonic acid was used instead of chlorosulfonic acid in Example 1.
[0054] In the first step, sulfamic acid, fluorosulfonic acid and thionyl chloride are reacted to give HN(SO 2 Cl)(SO 2 F). That is, the substance generated during the second step process of Example 1 was obtained in this step. This can be attributed to the sulfonation reaction with fluorosulfonic acid of the intermediate obtained by substituting chlorine for the OH group in sulfamic acid.
[0055] In the second step, potassium fluoride (KF) is added to the first intermediate product obtained in the first step from which excess thionyl chloride has been removed to allow a reaction to proceed. Thereby, almost the entire amount of by-products, namely sulfuric acid and hydrochloric acid, are converted into salts.
[0056] In the third step, water is added to the second intermediate product of the second step. Thus KN(S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com