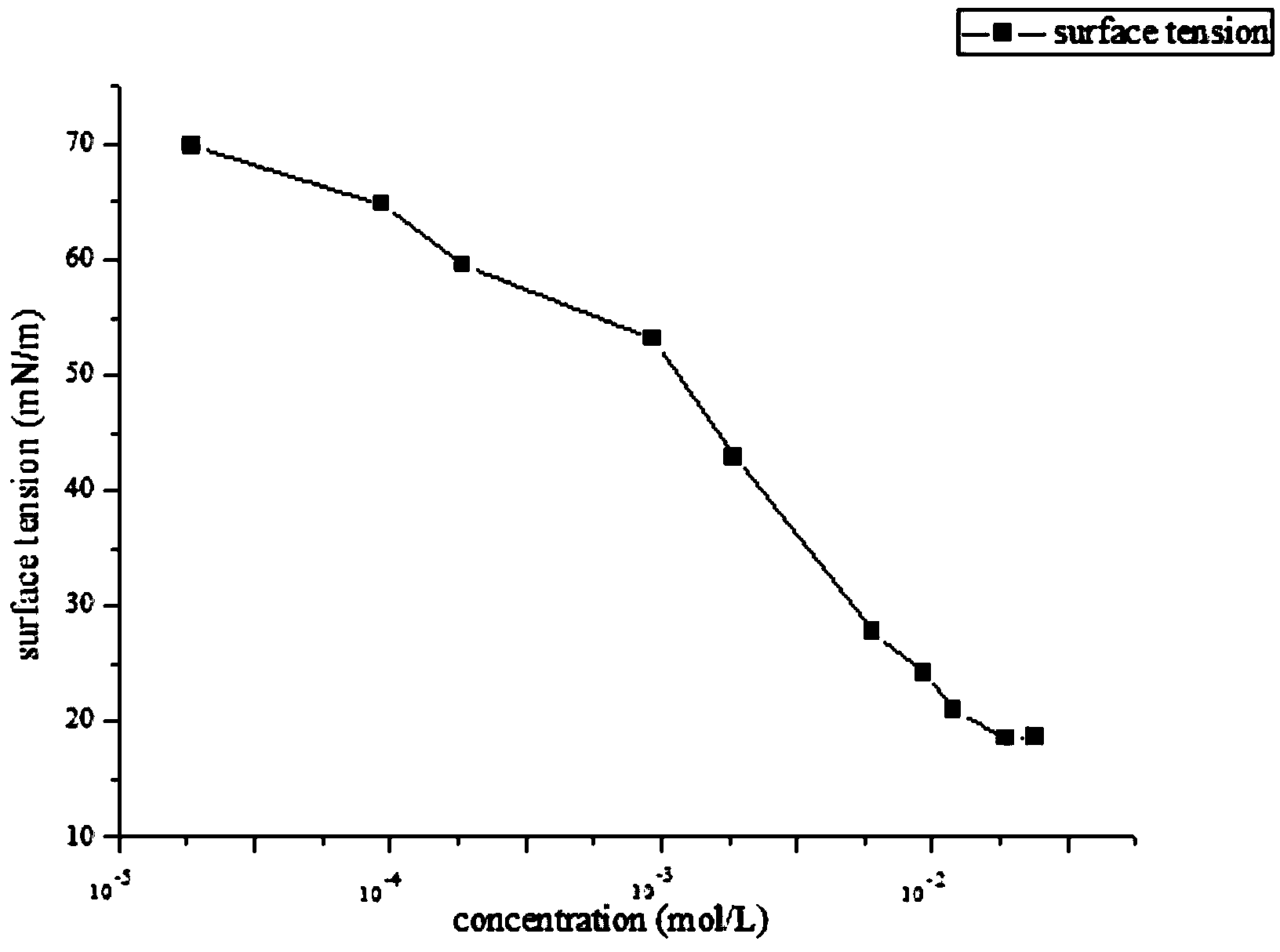
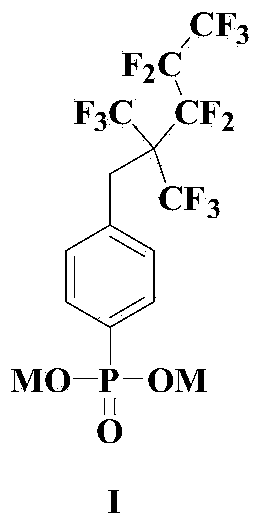
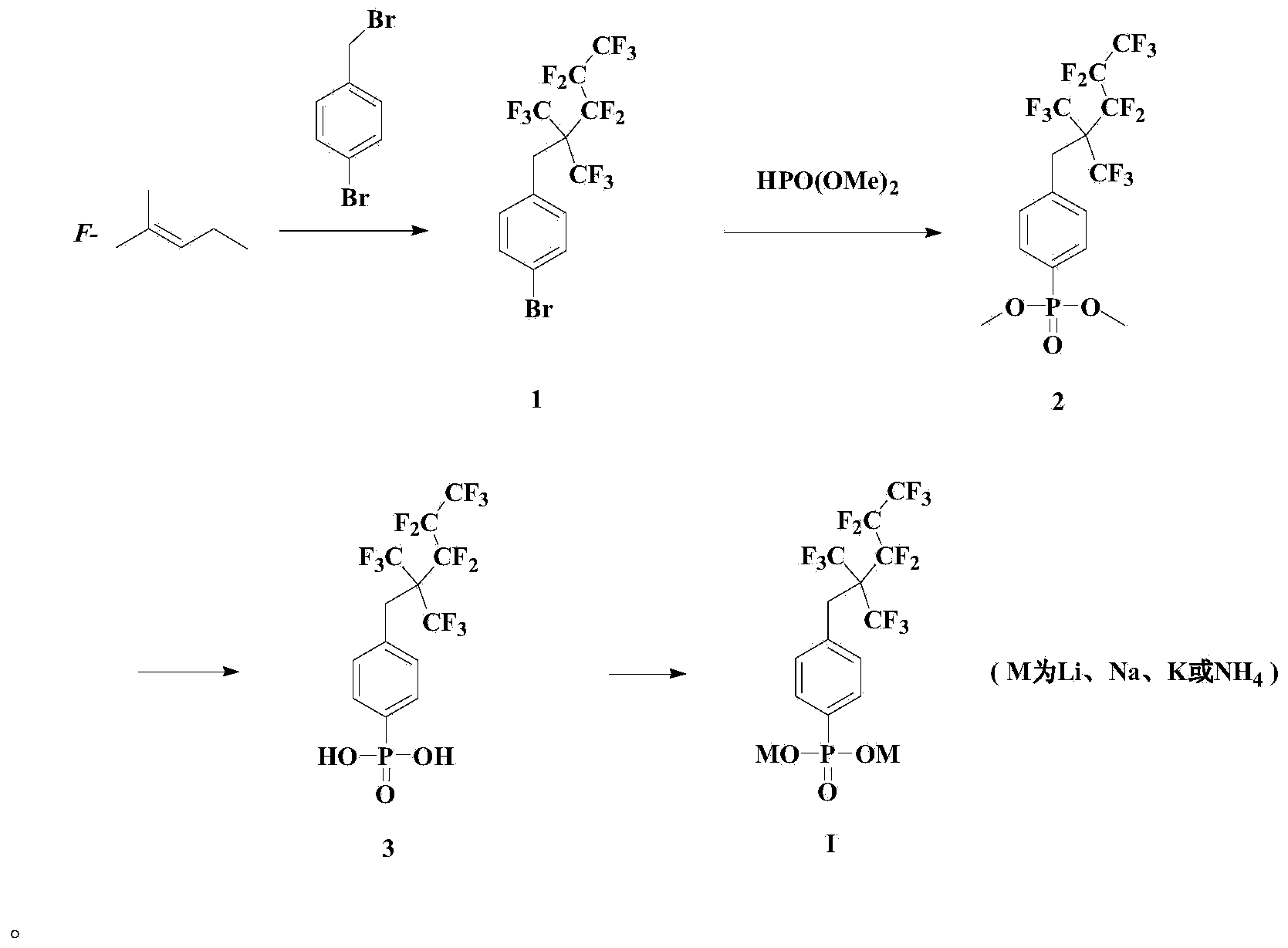
Anionic fluorocarbon surfactant and preparation method thereof
An anionic, fluorocarbon surface technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of application limitations, low surface activity of branched chain products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of fluorine-containing intermediate compound 1
[0035]
[0036]5g of p-bromobenzyl bromide, 12g of perfluoro-2-methyl-2-pentene, 2.32g of potassium fluoride and 30mL of DMF were successively added into a 50mL sealed tube, and reacted at 100°C for 24 hours. The system was cooled down to room temperature, and the mixed system was poured into diethyl ether. The organic phase was washed twice with deionized water, then washed with saturated brine, and finally dried with anhydrous sodium sulfate, filtered, and the solvent removed. The mixture was purified through a silica gel column with petroleum ether as the eluent to obtain 5.86 g of compound 1.
[0037] 1 H NMR (CDCl 3 ,300MHz): δ(ppm)3.50(s,CH 2 ,2H),7.12-7.22(d,J=8.4Hz,Ar-H,2H),7.40-7.50(d,J=8.4Hz,Ar-H,2H); 19 F NMR (CDCl 3 ,282MHz,):δ(ppm)-62.63~-62.85(m,6F),-80.40~-80.60(t,3F),-106.20~-106.60(m,2F),-123.30~-123.60(m, 2F).
Embodiment 2
[0038] Embodiment 2: the synthesis of fluorine-containing intermediate compound 2
[0039]
[0040] Add 0.046g tetrakis(triphenylphosphine) palladium to 50mL sealed tube, after three times of gas exchange, add 1.952g compound 1, 0.54mL HPO(OMe) under the protection of argon 2 , 0.83mL Et 3 N and 20mL of toluene, after the addition, the system was sealed and reacted at 100°C for 12 hours. The system was lowered to room temperature, ether was added, filtered, the filtrate was decompressed to remove the solvent, the mixture was purified by silica gel column, the polarity of the eluent was increased from PE:EA=1:1 to 1:4, and finally 1.55 g of colorless liquid was obtained 2.
[0041] 1 H NMR (CDCl 3 ,300MHz): δ(ppm)3.54(s,CH 2 ,2H),3.73(d,J=11.1Hz,6H),7.38(dd,J=7.8,3.4Hz,Ar-H,2H),7.72(dd,J=13.1,8.2Hz,Ar-H,2H ); 19 F NMR (CDCl 3 ,282MHz,):δ(ppm)-62.70~-62.90(m,6F),-80.50~-80.70(t,3F),-106.30~-106.60(m,2F),-123.40~-123.60(m, 2F); 31 P NMR (CDCl 3 ,121MHz):δ(ppm)22.27(...
Embodiment 3
[0042] Embodiment 3: the synthesis of fluorine-containing intermediate compound 3
[0043]
[0044] 5g of compound 2, 30mL of water and 20mL of concentrated hydrochloric acid were refluxed for two days. The product became a massive solid, which was filtered, washed with water, and then washed with chloroform, and the solvent was removed under reduced pressure to obtain 4.5 g of white solid 3.
[0045] 1 H NMR (DMSO-d 6 ,300MHz): δ(ppm)3.69(s,CH 2 ,2H),7.31~7.40(m,Ar-H,2H),7.58~7.70(m,Ar-H,2H); 19 F NMR (DMSO-d 6 ,282MHz,):δ(ppm)-62.60~-62.83(m,6F),-80.95~-81.15(t,3F),-106.50~-106.90(m,2F),-123.50~-123.80(m, 2F); 31 P NMR (DMSO-d 6 ,121MHz): δ(ppm)13.70(s); 13 C NMR (DMSO-d 6 ,100MHz): δ(ppm)31.56,110~130,130.41,131.46,132.86,133.76,134.67; IR(cm -1 ):3400~3600,1455.2;LRMS(EI):490,171.;HRMS(EI):m / z calcd for C 6 f 13 CH 2 C 6 h 4 PO(OH) 2 :490.0005,found490.0003.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com