Asymmetric synthesis method of optically pure tanshinol and derivative of tanshinol
A synthesis method and technology of derivatives, applied in the fields of organic synthesis and pharmaceutical synthesis, can solve the problems of high reaction pressure of catalyst hydrogenation method, unsuitable for large-scale synthesis, harsh reaction conditions, etc., and achieve mature synthesis method, high yield and selectivity. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
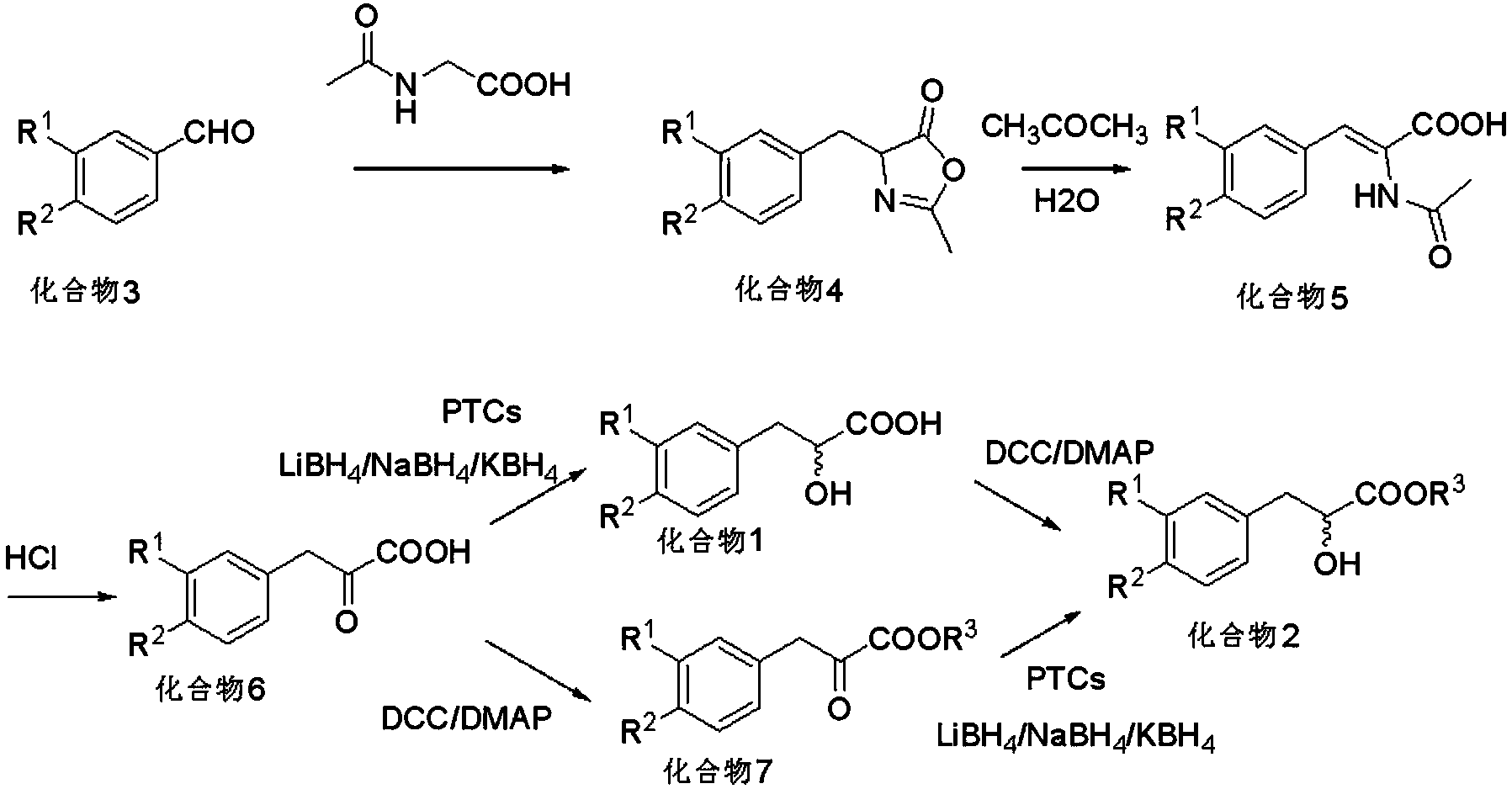
[0035] see figure 1 , the asymmetric synthesis method of above-mentioned optically pure Danshensu and its derivatives comprises the following steps:
[0036] (1) Using benzaldehyde or benzaldehyde derivatives (compound 3) as the starting material, the oxazolone compound (compound 4 );
[0037] (2) Oxazolone compounds (compound 4) were hydrolyzed into enamine compounds (compound 5) in acetone / water system;
[0038] (3) The enamine compound (compound 5) is further hydrolyzed in dilute hydrochloric acid to obtain the pyruvate compound (compound 6);
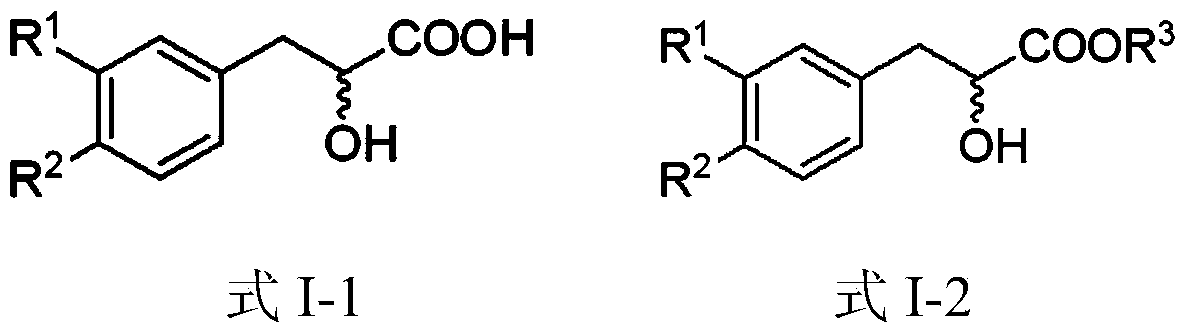
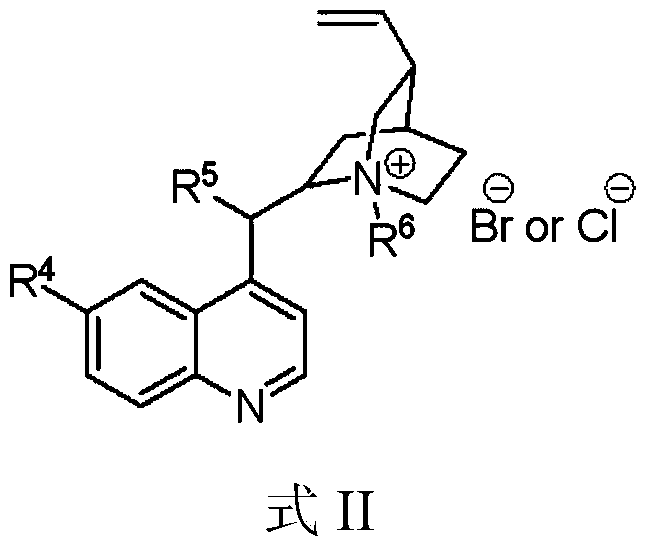
[0039] (4) Pyruvate compounds (compound 6) are reduced to α-hydroxypropionic acid compounds (compound 1) under phase transfer conditions, and the phase transfer catalysts are cinchona phase transfer catalysts (PTCs);
[0040] (5) Pyruvate compounds (compound 6) can also react with alcohols to form α-keto ester compounds (compound 7) under Steglich esterification reaction (DCC / DMAP) conditions;
[0041] (6) α-hydroxypropionic acid...
Embodiment 1
[0049] 1) Protection of 3,4-dihydroxybenzaldehyde (R 1 =R 2 =OH)
[0050] Add 200mL of N,N-dimethylformamide, 17.22g (0.126mol) of 3,4-dihydroxybenzaldehyde, and 86.8g (0.63mol) of potassium carbonate to a 500mL three-necked flask equipped with a constant pressure funnel and a reflux condenser. Add 36.3 mL (0.315 mol) of benzyl bromide to the constant pressure funnel; raise the temperature to 65°C, slowly drop in benzyl bromide (about 30 min), and continue the reaction for 6 h after the drop is complete. The reaction was detected by TLC. After the reaction was completed, it was filtered with suction, and the filtrate was distilled off under reduced pressure to remove N,N-dimethylformamide. The residue was poured into ice water to obtain a pale yellow precipitate, which was filtered and dried to obtain 38.3 g of a yellow solid (see formula 1 for the structure). , yield 96%. melting point, 1 The HNMR and MS data are consistent with those reported in the literature.
[0051]...
Embodiment 2
[0063] Step 1) to step 5) are the same as in Example 1;
[0064] 6) Isopropyl (R)-β-(3,4-methylenedioxyphenyl)-α-hydroxypropionate (Compound 2, R 1 =R 2 =OBn) synthesis
[0065] Add 1.88g (5mmol) of compound 1, 2.06g (10mmol) of N,N'-dicyclohexylcarbodiimide (DCC), 0.732g (6mmol) of 4-dimethyl to a 100mL three-necked flask Aminopyridine (DAMP), 1.5mL isopropanol, 40mL dichloromethane, stirred at room temperature for 24h. TLC detection reaction, after the reaction is completed, filter, add 50mL of water, after vigorous stirring, separate the organic phase, extract the water phase with ethyl acetate 3*20mL, combine the organic phase, dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure, 200 -300 mesh silica gel column chromatography to obtain 1.94 g of a white solid (see formula III for the structure), with a yield of 94%. melting point, 1 The HNMR and MS data are consistent with those reported in the literature.
[0066]
[0067] 7) Synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com