Preparation method for (6-Chloro-pyridazino-3-yl) formic acid
A technology of chloropyridazine and formic acid, which is applied in organic chemistry and other fields, can solve problems such as low chlorination efficiency, strong corrosion, and high toxicity, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
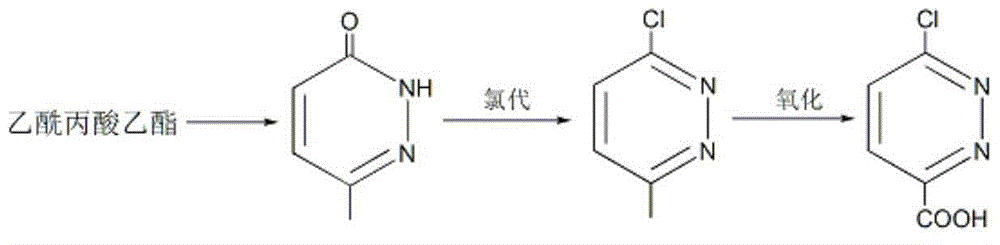
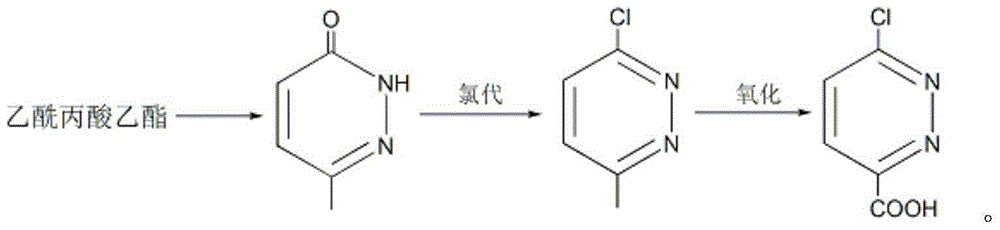
[0037] Embodiment 1: the preparation of 6-methyl-3 (2H)-pyridazinone
[0038] Put ethyl levulinate (43.3g, 0.3mol), 80wt% hydrazine hydrate (35g, 0.55mol), 10mL ethanol and 20g sodium hydroxide into the reaction flask in sequence, stir and heat to 85-90°C, and keep for 5 hours; Cool to room temperature, adjust the pH to weak alkaline with dilute hydrochloric acid, then add 300mL of toluene, stir for 30 minutes and let it stand; separate the toluene phase and dry it with anhydrous sodium sulfate, remove the desiccant, slowly add 20mL of liquid bromine, and then heat To 95 ~ 100 ℃, keep for 3 hours; add 100mL of glacial acetic acid, stir well, stand still, separate layers; separate the acetic acid layer, add 40g of sodium acetate, boil and keep for 1 hour; remove the solvent under reduced pressure, and cool the residue After that, colorless crystals were precipitated, and 33.2 g of 6-methyl-3(2H)-pyridazinone was obtained by suction filtration, m.p.: 130.2-130.8°C, and the yield...
Embodiment 2
[0042] Embodiment 2: Preparation of 3-chloro-6-methylpyridazine
[0043] Add 6-methyl-3(2H)-pyridazinone (55g, 0.5mol), tetramethylammonium chloride (49.5g, 0.45mol), and triphosgene (237.4g, 0.8mol) into the reaction flask in sequence; stir , heated to 145°C and kept for 5 hours; after the reaction solution was cooled to room temperature, it was slowly poured into about 1.5Kg of ice and stirred for 2 hours; phase, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, and dried with anhydrous sodium sulfate; the ethyl acetate was removed under reduced pressure to obtain 57.1 g of 3-chloro-6-methylpyridazine as a light yellow solid, m.p.: 48.6-50.0°C, yield 89%.
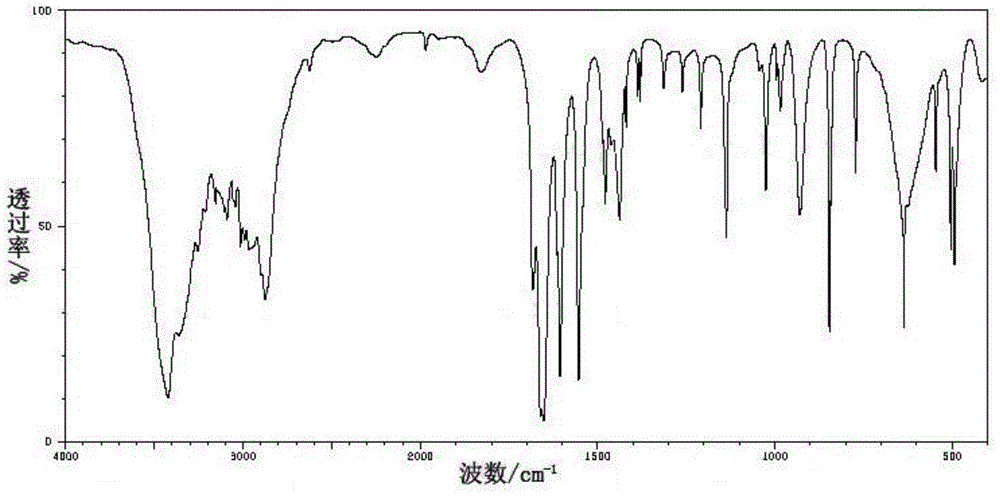
[0044] The Spectral Data of 3-Chloro-6-methylpyridazine
[0045] 1 HNMR (CDCl 3 ,ppm):δ7.664(d,1H);7.633(d,1H);2.281(s,3H).
Embodiment 3
[0046] Embodiment 3: Preparation of 6-chloropyridazine-3-carboxylic acid
[0047] Put 3-chloro-6-methylpyridazine (64.3g, 0.5mol) and 650mL cyclohexane into the reaction flask, stir and cool with ice water; %H 2 o 2 (79.4g, 0.7mol) solution was added within 40 minutes; the temperature was raised to 55°C and reacted for 2 hours; cooled to room temperature, the organic phase was separated and dried with anhydrous sodium sulfate; cyclohexane was removed under reduced pressure to obtain 56g6- Chlorpyridazine-3-carboxylic acid is a white solid, m.p.: 139.2-141.1°C, the yield is 71%.
[0048] Spectral data of 6-chloropyridazine-3-carboxylic acid: 1 HNMR (CDCl 3 ,ppm): δ8.424(d,1H);7.937(d,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com