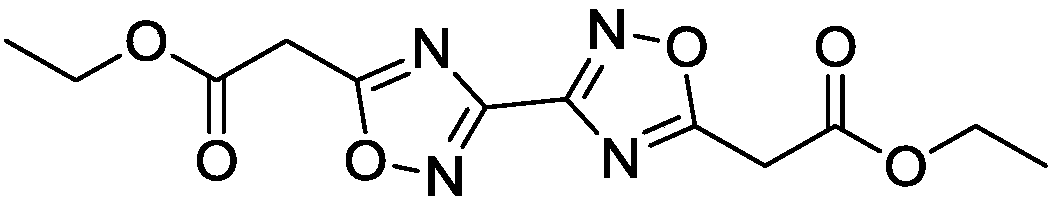
Synthesis of 5,5'-diacetoxyethyl-3,3'-bi-1,2,4-oxadiazole
An ethyl acetate-based, synthetic method technology, applied in organic chemistry and other directions, can solve problems such as poor selectivity and low yield, and achieve the effects of improving selectivity and reducing reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Synthesis of 5,5'-diacetoxyethyl-3,3'-bi-1,2,4-oxadiazole
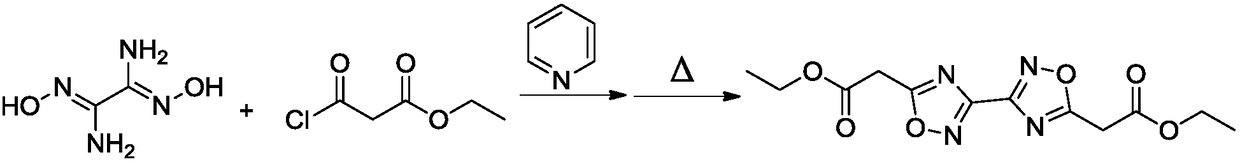
[0022] (1) Under the condition of 25°C, add 30ml of ethylene glycol dimethyl ether and 1.6g of pyridine into the reaction flask in turn, after stirring evenly, add 1.2g of diaminoglyoxime in batches, stir until completely dissolved, drop Add 3.0 g of ethyl chloroformoacetate, and react at 40°C for 30 min;
[0023] (2) Continue to heat the reaction solution obtained in step (1) to 100°C, and after continuing to react for 2 hours, pour the reaction solution into water, extract with chloroform, dry and distill under reduced pressure to obtain ethyl 5,5′-diacetate Base-3,3'-bi-1,2,4-oxadiazole was 1.92 g, and the yield was 62%.
[0024] Structure Identification:
[0025] Elemental analysis:
[0026] Molecular formula: C 12 h 14 N 4 o 6
[0027] Theoretical value: C 46.45, H 4.55, N 18.06;
[0028] Found: C 46.51, H 4.52, N 18.10.
[0029] Infrared (KBr, cm -1 ): 3473, 3376, 3180, 2976, 1751, 16...
Embodiment 2
[0034] (1) Under the condition of 25°C, add 25ml of ethylene glycol dimethyl ether and 0.4g of pyridine into the reaction flask in turn, after stirring evenly, add 1.2g of diaminoglyoxime in batches, stir until completely dissolved, drop Add 1.5 g of ethyl chloroformoacetate, and react at 30°C for 40 minutes;
[0035] (2) Continue to heat the reaction solution obtained in step (1) to 80°C, and after continuing the reaction for 3 hours, pour the reaction solution into water, extract with chloroform, dry and distill under reduced pressure to obtain ethyl 5,5′-diacetate Base-3,3'-bi-1,2,4-oxadiazole 1.86g, yield 60%.
Embodiment 3
[0037] (1) Under the condition of 25°C, add 40ml of ethylene glycol dimethyl ether and 2.0g of pyridine into the reaction flask in turn, after stirring evenly, add 1.2g of diaminoglyoxime in batches, stir until completely dissolved, drop Add 4.5 g of ethyl chloroformoacetate, and react at 40°C for 20 min;
[0038] (2) Continue to heat the reaction solution obtained in step (1) to 100°C, and after continuing to react for 2 hours, pour the reaction solution into water, extract with chloroform, dry and distill under reduced pressure to obtain ethyl 5,5′-diacetate Base-3,3'-bi-1,2,4-oxadiazole was 1.92 g, and the yield was 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com