Method for preparing 2'-deoxyuridine
A technology of deoxyuridine and uridine, which is applied in the field of medicine, can solve the problems of high environmental and human hazards and difficult waste disposal, and achieve the effects of shortening the production cycle, reducing equipment investment, and reducing labor costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
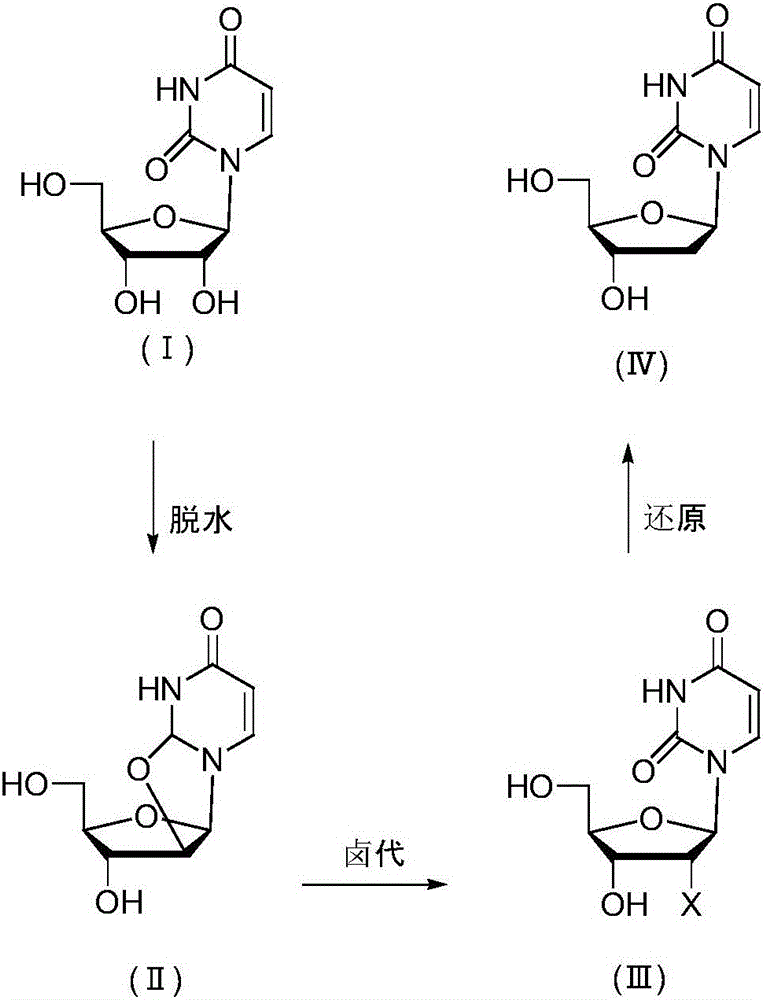
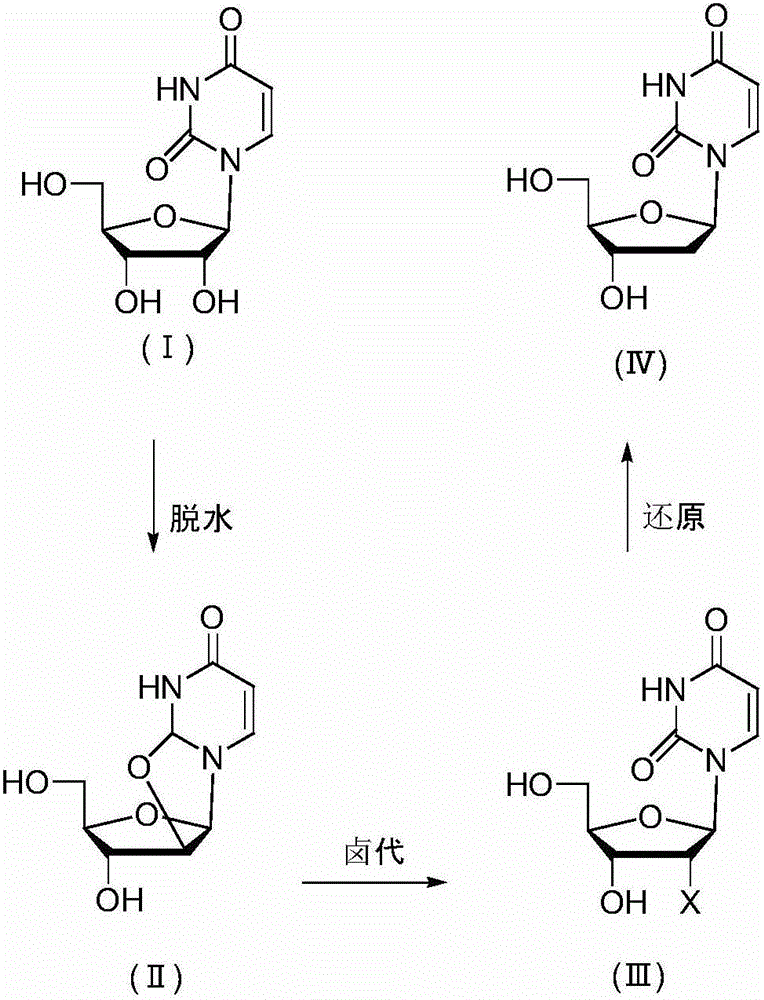
[0027] Embodiment 1 prepares 2'-deoxyuridine shown in formula (IV) in three steps
[0028] a, Preparation of uridine dehydrate
[0029] 100 g of uridine (compound of formula I), 105 g of diphenyl carbonate, 0.5 g of sodium hydroxide and 200 ml of N,N-dimethylformamide (DMF) were incubated at 140° C. for 12 hours. Evaporate the solvent to dryness, add 200ml of a mixed solvent of methanol and ethyl acetate, keep at 60°C for 2h, keep at 4°C for 2h to crystallize, filter and dry to obtain uridine dehydrate 92g, HPLC purity 99.5%.
[0030] b, Preparation of uridine nucleoside halides
[0031] 50 g of uridine dehydrate was added to a DMF solution of 200 g of HCl, and kept at 40° C. for 14 hours. Evaporate the solvent to dryness, add 100ml of water, keep at 4°C for 2h to crystallize, filter and dry to obtain 55g of uridine nucleoside halide, HPLC purity 98.9%.
[0032] c, Preparation of 2'-deoxyuridine
[0033] Add 5 g of nickel and 200 ml of methanol to 25 g of uridine halide, f...
Embodiment 2
[0034] Example 2 One-pot preparation of 2'-deoxyuridine shown in formula (IV)
[0035] 20 g of uridine (compound of formula I), 15 g of dimethyl carbonate, 0.2 g of sodium hydroxide and 100 ml of N,N-dimethylformamide (DMF) were incubated at 140° C. for 12 hours. A solution of 50 g of HCl in DMF was added. Incubate at 40°C for 15 hours. Add 5g of nickel and 200ml of methanol, feed hydrogen to maintain the pressure of hydrogen, slowly add a certain amount of 4mol / L sodium hydroxide dropwise, and keep the temperature at 40°C for 20 hours. The liquid was evaporated to dryness, and 20 g of water was added to the solid, crystallized at 4°C, recrystallized from methanol, and dried to obtain 16.1 g of 2'-deoxyuridine (compound of formula IV), with an HPLC purity of 99.0%.
Embodiment 3 1
[0036] Example 3 One-pot method for preparing 2'-deoxyuridine (compound of formula IV)
[0037] 20 g of uridine (compound of formula I), 20 g of diethyl carbonate, 2 g of sodium bicarbonate and 100 ml of DMF were incubated at 90° C. for 3 hours. A solution of 60 g of HCl in DMF was added. Incubate at 50°C for 24 hours. Add 10g of nickel and 200ml of methanol, pass in hydrogen, keep the pressure of hydrogen, slowly add a certain amount of 4mol / L sodium hydroxide dropwise, and keep the temperature at 50°C for 5 hours. The liquid was evaporated to dryness, and 20 g of water was added to the solid, crystallized at 4°C, recrystallized from methanol, and dried to obtain 15.0 g of 2'-deoxyuridine (compound of formula IV), with an HPLC purity of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com