Green catalytic synthesis method for N-(phenylimino)indazole-1-thioamides
A technology of phenylimino and thioamide, applied in organic chemistry and other directions, can solve the problems of easy hydrolysis of ionic liquids, unsatisfactory catalytic efficiency, unsuitable for biodegradation, etc., and achieves easy industrialized large-scale production and less catalyst usage. , the effect of not easy to hydrolyze
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
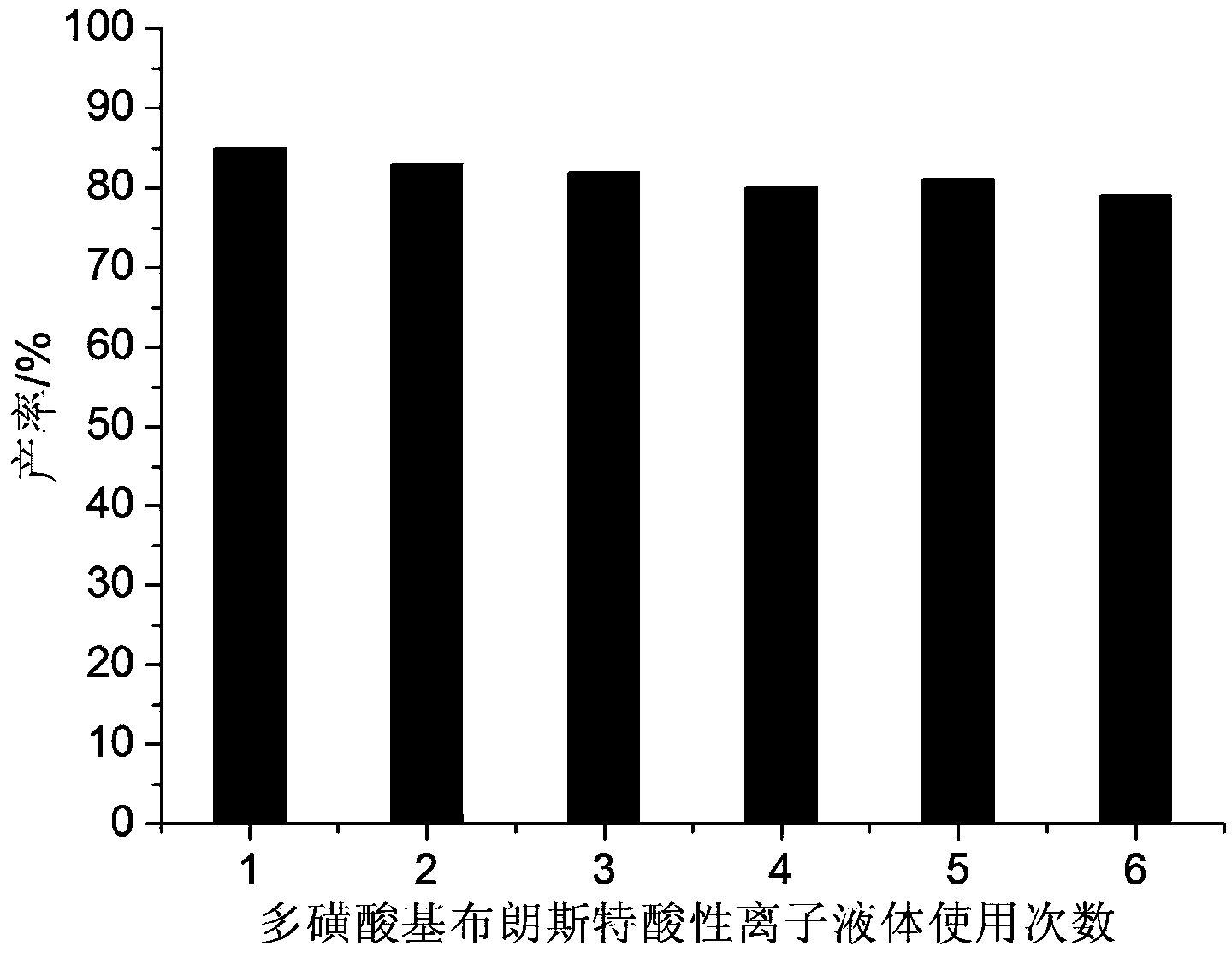
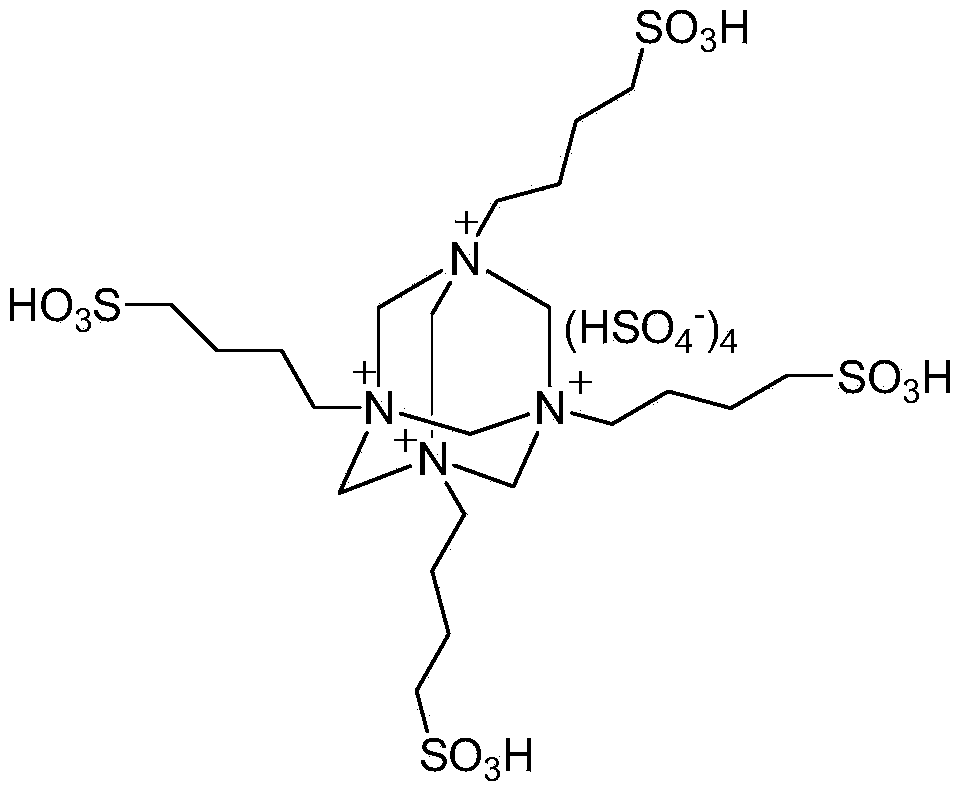
[0022] Example 1: 2mmol of benzaldehyde, 2mmol of dithizone, 2mmol of 5,5-dimethyl-1,3-cyclohexanedione and 0.6mmol of BAIL were added to a 25ml single-necked flask equipped with a stirring bar and a condenser. React under vigorous stirring at 80°C for 35 minutes, TLC (thin-plate chromatography) tracking detection (developing solvent is n-hexane: ethyl acetate = 4:1), the raw material point disappears, ice water cooling, suction filtration, the filter residue is passed through a silica gel chromatography column (flow The phase is n-hexane:ethyl acetate=4:1) to obtain pure 2,3,4,5,6,7-hexahydro-6,6-dimethyl-4-oxo-2,3-diphenyl Base-N-(phenylimino)indazole-1-thioamide, the yield was 85%. The filtrate (the main components are BAIL and water) can be reused after successively rotary evaporation and vacuum drying.
[0023] 2,3,4,5,6,7-Hexahydro-6,6-dimethyl-4-oxo-2,3-diphenyl-N-(phenylimino)indazole-1-sulfur Substituted amides: m.p.186~188℃; 1 H NMR (400MHz, CDCl3): δ=0.78(s, 3H),...
Embodiment 2
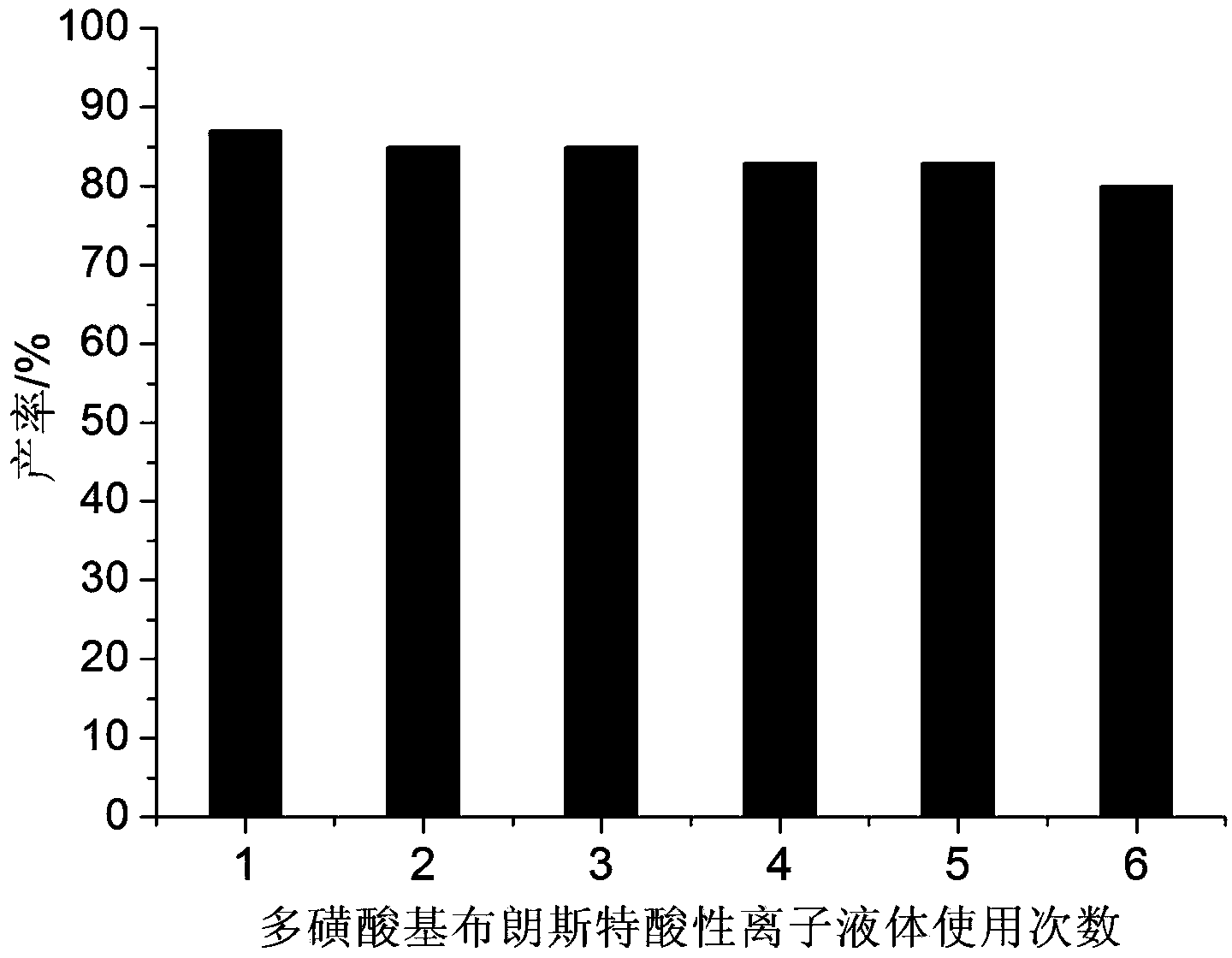
[0024] Example 2: 2mmol p-chlorobenzaldehyde, 2mmol dithizone, 2mmol 5,5-dimethyl-1,3-cyclohexanedione and 0.7mmol BAIL were added to a 25ml single-necked bottle with a stirring bar and a condenser . React under vigorous stirring at 90°C for 40 minutes, TLC (thin-plate chromatography) tracking detection (developing solvent is n-hexane: ethyl acetate = 4:1), the raw material point disappears, ice water cooling, suction filtration, the filter residue is passed through a silica gel chromatography column (flow The phase is n-hexane:ethyl acetate=4:1) to obtain pure 3-(4-chlorophenyl)-2,3,4,5,6,7-hexahydro-6,6-dimethyl- 4-Oxo-2-phenyl-N-(phenylimino)indazole-1-thioamide, the yield was 82%. The filtrate (the main components are BAIL and water) can be reused after rotary evaporation and vacuum drying.
[0025] 3-(4-Chlorophenyl)-2,3,4,5,6,7-hexahydro-6,6-dimethyl-4-oxo-2-phenyl-N-(phenylene Amino) indazole-1-thioamide: m.p.87~89℃; 1 H NMR (400MHz, CDCl 3): δ=0.80(s, 3H), 0.95(s,...
Embodiment 3
[0026] Example 3: 2mmol p-phenylbenzaldehyde, 2mmol dithizone, 2mmol5,5-dimethyl-1,3-cyclohexanedione and 0.6mmol BAIL were added to a 25ml single-necked bottle with a stirring bar and a condenser middle. React under vigorous stirring at 95°C for 40 minutes, TLC (thin plate chromatography) follow-up detection (developing solvent is n-hexane: ethyl acetate = 4:1), the raw material point disappears, ice water cooling, suction filtration, the filter residue is passed through a silica gel chromatography column (flow The phase is n-hexane:ethyl acetate=4:1) to obtain pure 3-(4-diphenyl)-2,3,4,5,6,7-hexahydro-6,6-dimethyl-4 -Oxo-2-phenyl-N-(phenylimino)indazole-1-thioamide, the yield was 81%. The filtrate (the main components are BAIL and water) can be reused after successively rotary evaporation and vacuum drying.
[0027] 3-(4-diphenyl)-2,3,4,5,6,7-hexahydro-6,6-dimethyl-4-oxo-2-phenyl-N-(phenylimino ) Indazole-1-thioamide: m.p.179~181℃; 1 H NMR (400MHz, CDCl 3 ): δ=0.84(s, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com