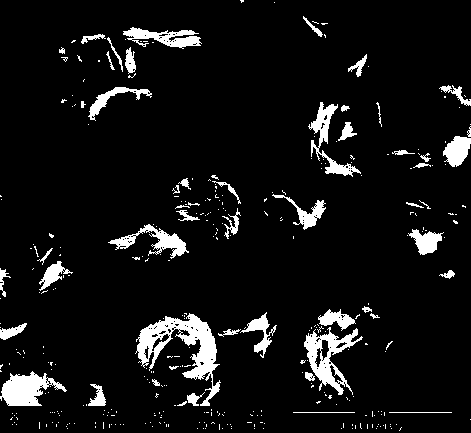Cobaltosic oxide powder and preparation method thereof
A technology of cobalt tetroxide powder and cobalt tetroxide, which is applied in chemical instruments and methods, nitrous oxide capture, cobalt oxide/cobalt hydroxide and other directions, can solve the problems of few researches on catalytic oxidation at room temperature, and achieve easy popularization and application, cheap and easy raw materials. obtainable, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Described in accordance with the technical scheme and process flow. Dissolve 1.245g of cobalt acetate in 50mL of water, then add 1mL of ethanolamine dropwise to the above solution to obtain a blue precipitate α-Co(OH) 2 , the mixture was placed in an ultrasonic reactor with circulating cooling water, the ultrasonic power was 150W, the ultrasonic frequency was 53KHz, the water temperature was maintained at 25°C, and the reaction was carried out for 2h. The cobalt hydroxide prepared above was sucked and dried in an oven at 80°C. The dried cobalt hydroxide was sintered at 200° C. for 1 h. The XRD diffraction pattern of the prepared tricobalt tetroxide is as follows figure 1 As shown, cobalt tetroxide can be obtained by sintering at 200 °C. Its surface morphology is as Figure 2b and Figure 2e As shown, it can be seen that its surface morphology is flake or flower shape. The specific surface area of the prepared cobalt tetroxide is shown in Table 1. At the sinterin...
Embodiment 2
[0044] Repeat Example 1, but the sintering temperature is 150°C, and its surface morphology is as Figure 2a and Figure 2d As shown, it can be seen that its surface morphology is flake or flower shape. The specific surface area of the prepared cobalt tetroxide is shown in Table 1. At the sintering temperature of 150°C, the BET specific surface area is as high as 140m 2 g -1 .
Embodiment 3
[0046] Repeat Example 1, but the sintering temperature is 250 ° C, and its surface morphology is as Figure 2c and Figure 2f As shown, it can be seen that its surface morphology is flake or flower shape. The specific surface area of the prepared cobalt tetroxide is shown in Table 1. At the sintering temperature of 250°C, the BET specific surface area is as high as 134m 2 g -1 .
[0047] Table 1. Specific surface area parameters of cobalt tetroxide obtained at different sintering temperatures.
[0048]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com