Beta-elemene substituted ethyl peracetylated sugar complex, beta-elemene substituted ethyl sugar complex, and preparation methods and use
A technology of fully acetylated sugar and full acetylation, which is applied in the field of medicine, can solve the problems of difficult absorption and low water solubility, and achieve the effects of enhanced biological activity, less stimulating effect on the body, and improved immunity of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
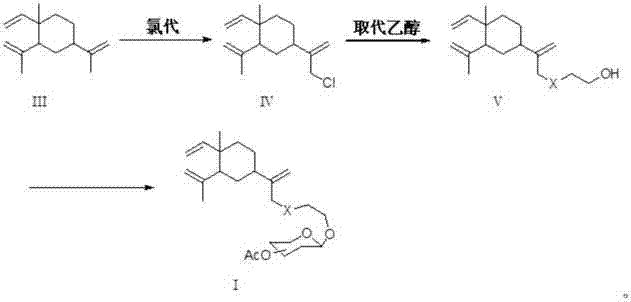
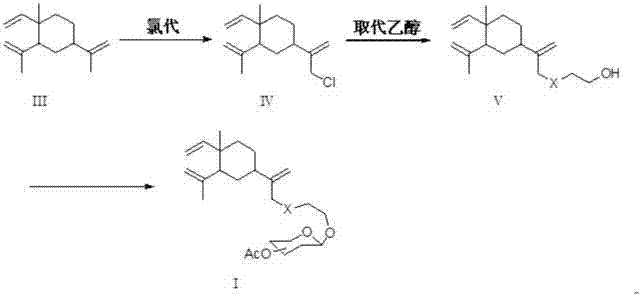
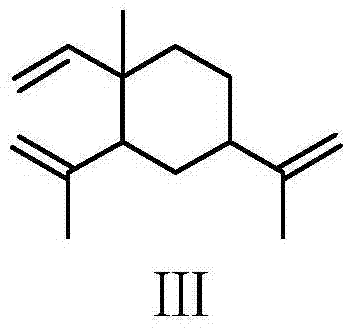
[0040] Preparation of β-elemene in place of ethanol:
[0041]
[0042] Weigh 50 g (0.245 mmol) of β-elemene into a 500 mL container with dichloromethane as the solvent. Weigh 41mL (0.735mmol) of glacial acetic acid into a constant pressure dropping funnel and dilute with dichloromethane. Weigh 108.5 grams of sodium hypochlorite solution (available chlorine ≥ 8.0%) in another constant pressure dropping funnel, and dilute with dichloromethane. Cool the container containing β-elemene-dichloromethane in an ice bath, and slowly add glacial acetic acid and sodium hypochlorite dropwise into the container containing β-elemene under vigorous stirring. After dropping, the reaction was continued for 6 hours. The progress of the reaction was checked by thin layer chromatography (TLC). After the reaction stopped, the reactant was transferred to a separatory funnel, and the water layer was separated, and the organic layer was washed with saturated brine and saturated sodium bicarbonat...
Embodiment 2
[0047] Weigh 0.505 g (1.015 mmol) of 2,3,4,6-O-tetraacetyl-α-D-galactose trichloroacetimidate into a 50 mL dry container, add 0.340 g (1.218 mmol) successively for implementation The β-elemene mercaptoethanol that example 1 prepares, 0.45 gram 4A molecular sieves, N 2Under protection, an appropriate amount of dry dichloromethane was added as a solvent. Cool in an ice-salt bath, and slowly add 0.318 mL (1.523 mmol) of BF under stirring 3 ·Et 2 O dichloromethane solution, after the dropwise addition was completed, the reaction was continued for 2 hours after being incubated, and the reaction process was detected by TLC. After the reaction was complete, triethylamine was added to terminate the reaction. Suction filtration with diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain a nearly colorless syrup, which was separated and purified by flash silica gel column chromatography. 498 mg of β-elemene substituted ethyl peracetylated galactose co...
Embodiment 3
[0052] in N 2 Under protection, take 0.155 g (0.377 mmol) of tetraacetylbromogalactose in a 50 mL dry container, add 0.144 g of β-elemene mercaptoethanol, and add an appropriate amount of dry CHCl 3 dissolve. Stir at room temperature for 30 minutes. Under the condition of avoiding light, add the above-prepared Ag 2 CO 3 - Diatomaceous earth 0.356 g, reflux, TLC detection reaction progress (petroleum ether: ethyl acetate 3:1 development, 10% sulfuric acid-methanol color development), after about 10 hours, the reaction is basically complete. After the reaction was terminated, the diatomaceous earth was suction filtered, and the filtrate was concentrated under reduced pressure to obtain 0.339 g of a brown syrup crude product. Purified by flash silica gel column chromatography (petroleum ether: ethyl acetate ratio of 3:2), the colorless syrup product β-elemene substituted ethyl peracetylated galactose complex 0.174 g was obtained, with a yield of 75%. and NMR 1 H. 13 C and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com