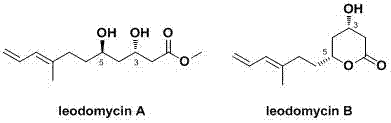
Method for stereoselective synthesis of IeodomycinA and B
A technology of stereoselectivity and synthesis method, applied in chemical instruments and methods, asymmetric synthesis, organic chemistry methods, etc., can solve the problems of long synthesis steps and achieve the effect of high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] In order to make the present invention clearer, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0030] 1. Synthesis of Compound 2
[0031] Dissolve oxalyl chloride (9 mL, 97.24 mmol) in dry dichloromethane (120 mL) at -78 °C, and then dissolve dimethyl sulfoxide (14 mL, 194.49 mmol) in dichloromethane ( 50 mL), mixed again, and after 15 min, geraniol (10.0 g, 64.83 mmol) was slowly added dropwise to the mixed solution within 30 min. Triethylamine (66 mL, 374.72 mmol) was dissolved in dry dichloromethane (80 mL), and the mixture was slowly added to the reaction flask, and then kept at -78°C for 1 h, and then The reaction bottle was placed in ice water until it was at room temperature overnight, and the plate was detected. After the reaction was detected, the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com