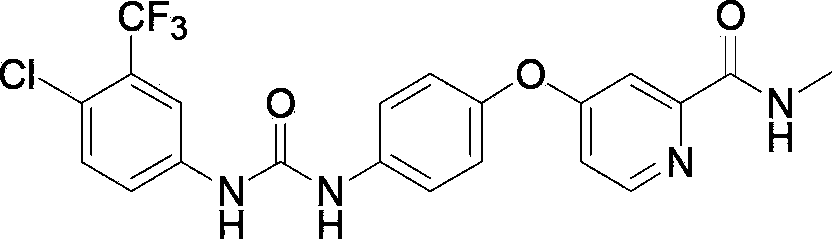
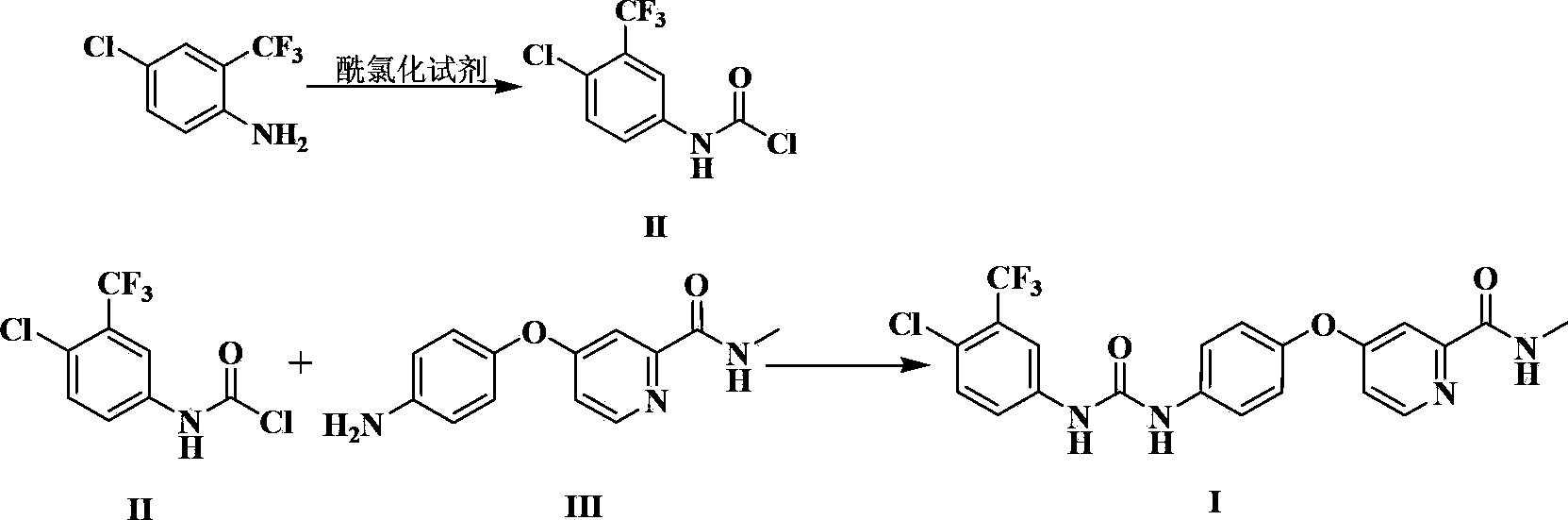
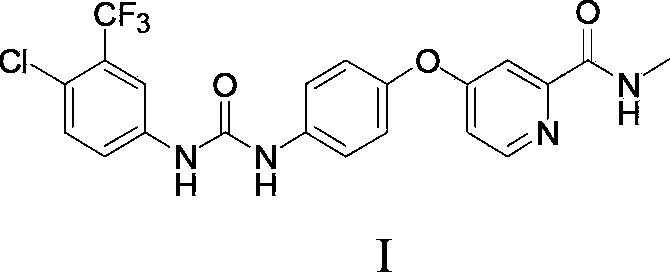
Preparation method of sorafenib
A technology of trifluoromethyl and aniline, applied in the field of medicine and chemical industry, can solve the problems of long reaction time, long production cycle, unsafety and the like, and achieve the effects of simplified operation steps, short reaction route and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 103ml of acetonitrile and 10.3g (34.8mmol) triphosgene to a 2000mL reaction flask, stir to dissolve, control the temperature to 10°C, add dropwise 20g (102.3mmol) of 4-chloro-3-trifluoromethyl-aniline, 200mL of acetonitrile and 14.5ml of triethylamine, after stirring for 3 hours, dropwise added 22.4g (92.0mmol) of compound III (ie 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide , the same below), 14.5ml of triethylamine and 224ml of acetonitrile mixture solution, temperature control to 10 ℃, stirring reaction for 3h, adding 500ml of water to the reaction system, stirring at room temperature for 2h, suction filtration, washing, drying to obtain 36g Rafenib, yield 84.3%, HPLC purity 98.6%.
Embodiment 2
[0047] Add 1L ethyl acetate and 100g (0.505mol) diphosgene to a 10L reactor, stir to dissolve, control the temperature to 20°C, add dropwise 194g (0.992mol) 4-chloro-3-trifluoromethyl-aniline, A mixed solution composed of 1.9L ethyl acetate and 141ml triethylamine was stirred for 2 hours; a mixed solution composed of 217g (0.893mol) compound III, 141ml triethylamine and 2.2L ethyl acetate was added dropwise, and the temperature was raised to 30°C. Stir the reaction for 2 hours; add 5.1L saturated saline to the reaction system, stir and wash, separate liquids, stir the organic phase at room temperature for 2 hours, suction filter, wash, and dry to obtain 355g of Sorafenib, with a yield of 85.5% and a purity of 98.3% by HPLC .
Embodiment 3
[0049] Add 1L tetrahydrofuran and 100g (0.337mol) triphosgene to a 10L reactor, stir to dissolve, cool down to 0°C, then dropwise add 194g (0.992mol) 4-chloro-3-trifluoromethyl-aniline, 1.9L tetrahydrofuran and a mixed solution composed of 141ml triethylamine, stirred and reacted for 4h. Then, a mixed solution consisting of 217g (0.893mol) of compound III, 2.2L of tetrahydrofuran and 141ml of triethylamine was added dropwise to the reaction solution, the temperature was controlled to 5°C, and the reaction was stirred for 4h. 5.1L of water was added to the reaction system, and stirred at room temperature for 2h. , Suction filtration, washing, drying, obtain 361g Sorafenib, yield 87.0%, HPLC purity 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com