Method for synthesizing quinazolino indazole derivatives
A synthesis method and quinazolinone technology are applied in the field of organic chemical synthesis and can solve problems such as environmental pollution and damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
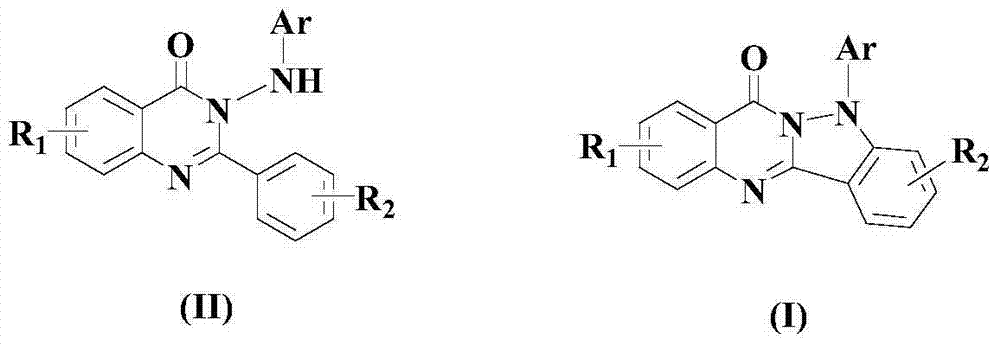
[0058] Preparation 1: 2-Phenyl-3-(phenylamino)quinazolin-4(3H)-one
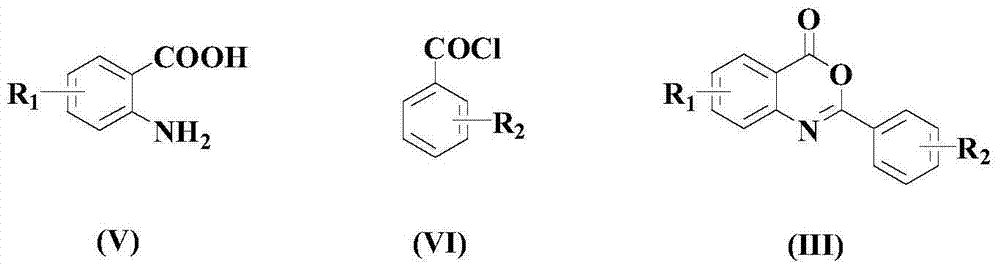
[0059] (1). Add 10mmol anthranilic acid and 20mmol sodium carbonate to a 100mL round bottom flask, then add 20mL THF, stir in an ice-water bath, add 25mmol benzoyl chloride, stir for 15 minutes, then warm to room temperature And continue to stir and react overnight, after the reaction is finished, add water to separate out the solid, filter, and the solid is rinsed with 50% methanol aqueous solution with a mass percentage concentration, and dried to obtain the product (formula (III) compound), the yield is 60%, and its melting point is 104-105°C. Its reaction formula is as follows:
[0060]
[0061] (2). Take 2mmol of the compound of formula (III) obtained in the previous step and 20mL of acetic acid into a 100mL round-bottomed flask, then add 2.5mmol of phenylhydrazine, reflux at 160°C overnight, add 30mL of water after cooling, and let the solution settle until the solution is clear. The solution was p...
preparation example 2
[0066] Preparation 2: 2-Phenyl-3-(p-tolylamino)quinazolin-4(3H)-one
[0067] Take 2mmol of the compound of formula (III) obtained in Preparation Example 1 step (1) and 15mL of acetic acid into a 100mL round-bottomed flask, then add 1.5mmol of p-methylphenylhydrazine, reflux at 140°C overnight, cool and add 25mL of water, and let it stand for precipitation To the solution clarification, the solution is poured out, and the precipitate is added with ethanol for recrystallization, filtered, rinsed with ethanol, and dried to obtain the product 2-phenyl-3-(p-tolylamino)quinazolin-4(3H)-one ( Formula (II) compound), yield 60%. Its reaction formula is as follows:
[0068]
[0069] Melting point: 181-182°C;
[0070] NMR: 1 HNMR (500Mz, DMSO-d 6 )δ8.94(s,1H),8.14(d,J=8.0Hz,1H),7.93-7.90(m,1H),7.80(d,J=8.0Hz,1H),7.75-7.73(m,2H ),7.60-7.57(m,1H),7.46-7.39(m,3H),6.92(d,J=8.5Hz,2H),6.49(d,J=8.5Hz,2H),2.14(s,3H) .
[0071] 13 CNMR (125Mz, DMSO-d 6 )δ 160.6, 157.5, 146.8, 144.7, 134...
preparation example 3
[0072] Preparation 3: 2-Phenyl-3-(p-trifluoromethylanilino)quinazolin-4(3H)-one
[0073] Except that p-methylphenylhydrazine was replaced by p-trifluoromethylphenylhydrazine, this preparation example 3 was implemented in the same manner as preparation example 2, and the product 2-phenyl-3-(p- Trifluoromethylanilino)quinazolin-4(3H)-one (compound of formula (II)), yield 62%. Its structural formula is as follows:
[0074]
[0075] Its melting point and NMR are as follows:
[0076] Melting point: 211-212°C;
[0077] NMR: 1 HNMR (500Mz, DMSO-d 6 )δ9.64(s,1H),8.17(d,J=8.0,1.0Hz,1H),7.95-7.92(m,1H),7.82(d,J=8.0Hz,1H),7.72-7.71(m ,2H), 7.63-7.60(m,1H), 7.48-7.41(m,5H), 6.78(d,J=8.5Hz,2H).
[0078] 13 CNMR (125Mz, DMSO-d 6 )δ 160.3, 157.1, 150.2, 146.7, 135.1, 133.9, 129.9, 128.7 (2C), 127.8, 127.6 (2C), 127.3, 126.3 (2C), 123.5, 120.1, 119.8, 119.5, 112.1 (2C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com