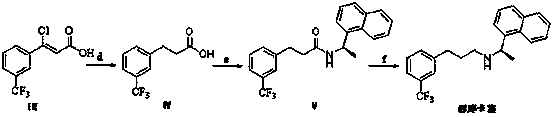
Preparation method of cinacalcet intermediate
A cinacalcet, Chinese-style technology, applied in the field of preparation of cinacalcet intermediates, can solve the problems of unsuitability for industrial production, carcinogenicity of ethyl acrylate, instability, etc., and achieves low cost, easy purification, and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
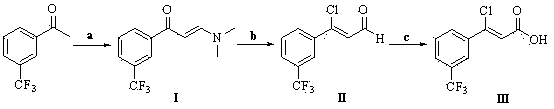
[0045] Preparation of (Z)-3-chloro-3-[3-(trifluoromethyl)phenyl]-2-propenoic acid
[0046] Dissolve 8.46g of 3-(trifluoromethyl)acetophenone and 21.3g of N,N-dimethylaminoformal in 100mL of DMF, and stir the reaction mixture under reflux at 153°C. TLC monitors that the reaction is complete, and evaporates the solvent Obtained 10.28g (E)-3-(N,N-dimethylamino)-1-[3-(trifluoromethyl)phenyl]-2-propen-1-one (compound of formula I), yield 94.0 %.
[0047] Dissolve 2.67g of the compound of formula I and 3.34g of phosphorus oxychloride in 25mL of dichloromethane, stir the reaction mixture at reflux temperature, monitor the completion of the reaction by TLC, remove the solvent to obtain a crude product, dissolve it in a mixture of 50mL of water and tetrahydrofuran solution (volume ratio = 1:1), stirred at room temperature for 24 hours, added water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated to give 2.52g (Z)-3-chloro-3-[3-(trifluoroform Base) ph...
Embodiment 2
[0050] Preparation of (Z)-3-chloro-3-[3-(trifluoromethyl)phenyl]-2-propenoic acid
[0051] Dissolve 8.46g of 3-(trifluoromethyl)acetophenone and 15.75g of N,N-dimethylaminopropyl acetal in 100mL of dioxane, and stir the reaction mixture under reflux at 101°C. TLC monitors that the reaction is complete. The solvent was evaporated to obtain 10.01g (E)-3-(dimethylamino)-1-[3-(trifluoromethyl)phenyl]prop-2-en-1-one (compound of formula I), yield 91.5 %.
[0052] Dissolve 2.67g of the compound of formula I and 6.86g of phosphorus pentachloride in 50mL of tetrahydrofuran, stir at 60°C, monitor the completion of the reaction by TLC, remove the solvent to obtain a crude product, and dissolve the crude product in a mixture of 50mL of water and acetone (volume ratio = 1 : 1), stirred at room temperature for 24h, added water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated to give 2.38g (Z)-3-chloro-3-[3-(trifluoromethyl)phenyl]- 2-Acrolein (compound o...
Embodiment 3
[0055] Preparation of (Z)-3-chloro-3-[3-(trifluoromethyl)phenyl]-2-propenoic acid
[0056] Dissolve 8.46g of 3-(trifluoromethyl)acetophenone and 19.84g of N,N-dimethylaminoacetal in 100mL of acetonitrile, and stir the reaction mixture under reflux at 80°C. TLC monitors that the reaction is complete, and evaporates the solvent 10.09 g of (E)-3-(dimethylamino)-1-[3-(trifluoromethyl)phenyl]prop-2-en-1-one (compound of formula I) was obtained with a yield of 92.3%.
[0057] Dissolve 2.67g of the compound of formula I and 6.69g of phosphorus oxychloride in 50mL of toluene, stir at 80°C, monitor the complete reaction by TLC, remove the solvent to obtain the crude product, and dissolve the crude product in a mixture of 50mL of water and acetonitrile (volume ratio = 2 : 1), stirred at room temperature, added water, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated to give 2.41g (Z)-3-chloro-3-[3-(trifluoromethyl)phenyl]-2 - Acrolein (compound of form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com