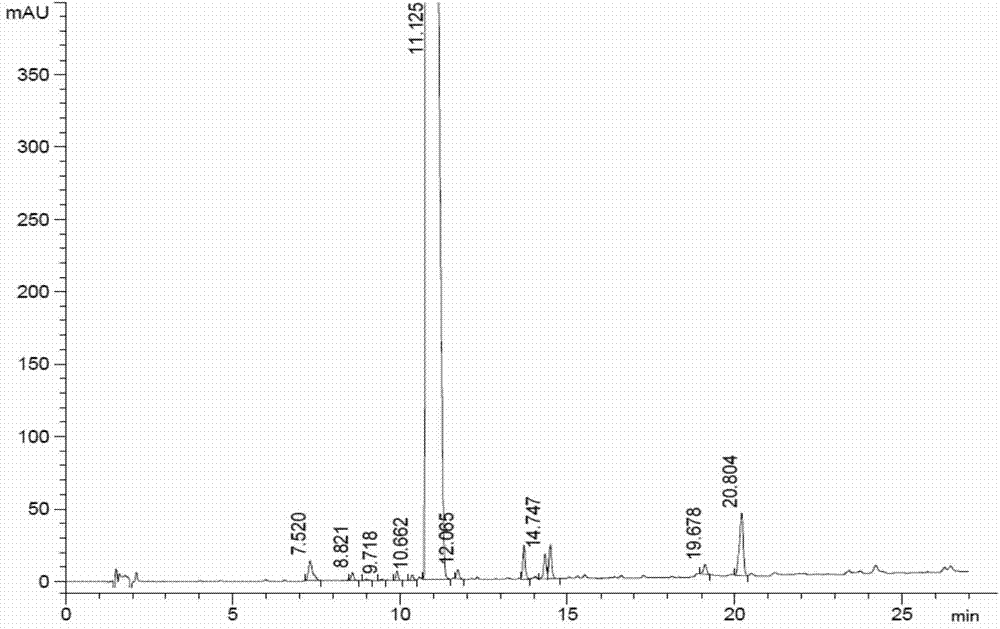
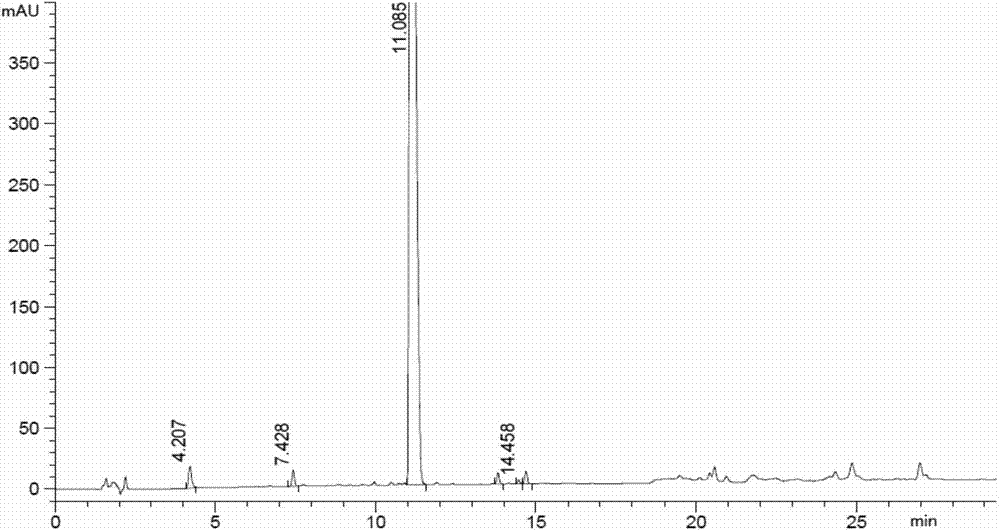
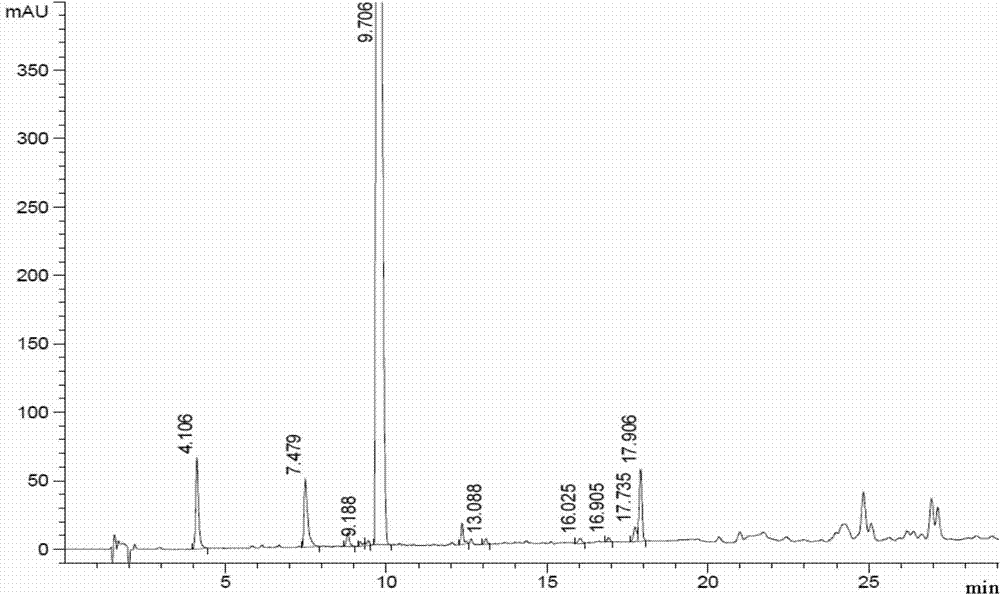
A kind of synthetic method of carfilzomib
A synthetic method, the technology of carfilzomib, which is applied in the field of carfilzomib synthesis, can solve the problems of multiple starting materials, complex and diverse reaction conditions, and poor controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] The preparation of embodiment 1 compound V
[0128] Under nitrogen protection, add 14.6g (0.10mol) of morpholin-4-ylacetic acid and 20.3g (0.105mol) of L-homophenylalanine methyl ester into a 1000mL three-necked flask, add 500mL of acetonitrile, and then add DIEA51 .7 g (0.4 mol), the mixture was cooled to 0°C with stirring. To this mixture was added HOBT 14.9 g (0.11 mol) followed by three additions of PyBOP totaling 57.3 g (0.11 mol) over five minutes. The reactant was placed under nitrogen, stirred and reacted for 8 hours, distilled under reduced pressure, the residue was dissolved in 300mL ethyl acetate, washed twice with saturated sodium bicarbonate, water and saturated saline respectively, each dosage was 150mL, organic The layer was evaporated to dryness under reduced pressure to obtain 19.5g of the compound, which was dissolved in a mixed solution of methanol:water=3:1, cooled to 0°C, and 12.1g (0.5mol) of lithium hydroxide was added to react for 12h, and then ...
Embodiment 2
[0132] The preparation of embodiment 2 compound V
[0133] Under the protection of argon, add 14.6g (0.10mol) of morpholin-4-ylacetic acid and 48.3g (0.25mol) of L-homophenylalanine ethyl ester into a 2000mL three-necked flask, add 1200mL tetrahydrofuran, and then add 158.2 g (2.0 mol) of pyridine, and the mixture was cooled to -20°C with stirring. Add HATU380.2g (1.0mol) to this mixture, place the reactant under nitrogen, stir the reaction for 24h, distill under reduced pressure, dissolve the residue in 300mL ethyl acetate, wash with saturated sodium bicarbonate, water and saturated saline successively Wash twice respectively, each time the amount is 150mL, the organic layer is evaporated to dryness under reduced pressure to obtain 21.6g of the compound, which is dissolved in a mixed solution of methanol: water = 9:1, cooled to 0°C, and 24.2g of lithium hydroxide is added (1.0mol) reacted for 8 hours, terminated the reaction with 200mL saturated ammonium chloride, adjusted t...
Embodiment 3
[0138] Embodiment 3 Preparation of Compound V
[0139] Add 14.6g (0.10mol) of morpholin-4-ylacetic acid and 114.8g (0.50mol) of L-homophenylalanine methyl ester hydrochloride into a 2000mL three-necked flask, add 600mL of DMF, and then add 50.6 g (0.50mol), the mixture was stirred at 20°C, TBTU64.2g (0.2mol) was added to the mixture, the reactant was placed under nitrogen, stirred for 24h, distilled under reduced pressure, and the residue was dissolved in 300mL of dichloro Methane was washed twice with saturated sodium bicarbonate, water and saturated brine successively, each time the dosage was 150mL, and the organic layer was evaporated to dryness under reduced pressure to obtain 20.4g of the compound, which was dissolved in methanol:water=5:1 mixed In the solution, cool to 0°C, add 19.4g (0.8mol) of lithium hydroxide to react for 8 hours, stop the reaction with 200mL saturated ammonium chloride, adjust the pH value to about 3 with 1N hydrochloric acid, extract twice with 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com