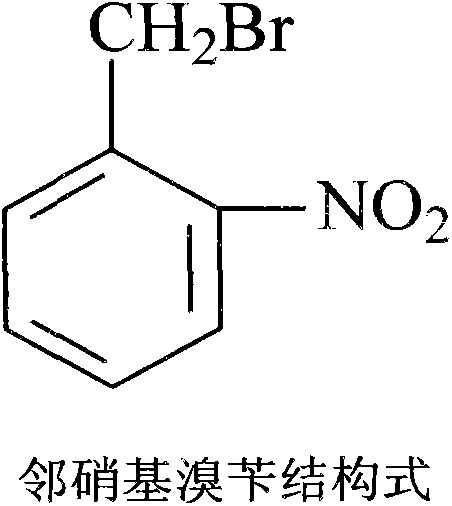
Production method for 2-nitrobenzyl bromide
The technology of an o-nitrobenzyl bromide and a production method is applied in the production field of the o-nitrobenzyl bromide, can solve the problems of large environmental pollution, high production cost, low yield and the like, achieves simple post-processing, improves reaction conversion rate, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
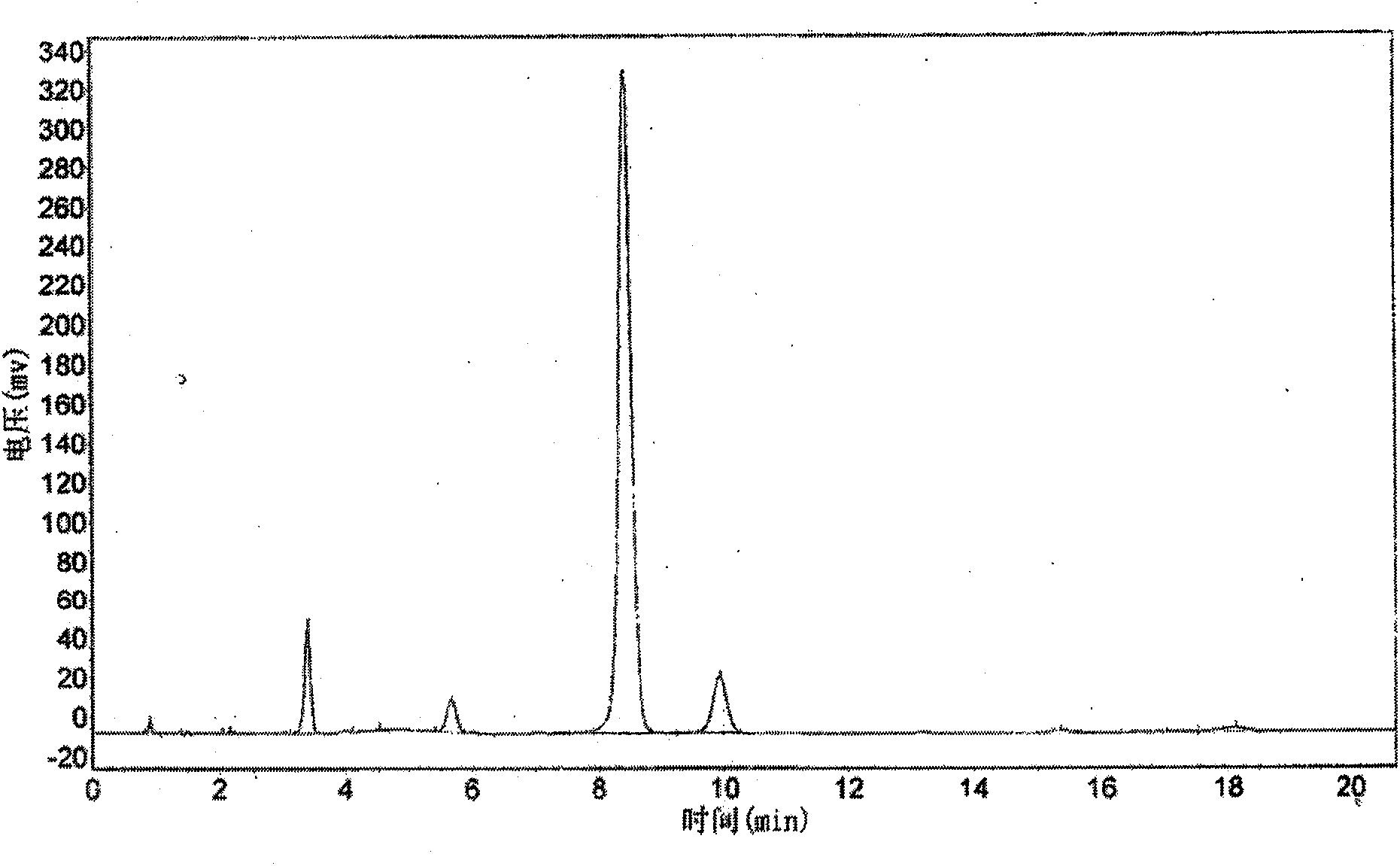
[0021] In a 1000ml three-necked flask, add 250g of water, 100g of o-nitrotoluene, PEG6002g, 110g of HBr with a mass concentration of 48%, and 10g of azobisisobutyronitrile, and dropwise add hydrogen peroxide with a mass concentration of 30% at 50-55°C 80g, after dripping for about 1 hour, keep it warm at this temperature for 6 hours until the redness fades away, remove the upper water layer, wash the lower layer with 5% sodium sulfite, then wash with water, add 100ml petroleum ether, stir and heat up Dissolved; after dissolving, the petroleum ether solution was lowered to room temperature, and the solution was layered. Petroleum ether was rinsed to obtain o-white nitrobenzyl bromide solid with a content of 80.7% and a yield of 80.2%. refer to figure 1 , the mobile phase is 80:20=methanol:water, the wavelength is 254nm, and the flow rate is 0.81 / h. The peak with a retention time of 8.4 min is o-nitrobenzyl bromide; the peak with a retention time of 9.9 min is o-nitrobenzyl br...
Embodiment 2
[0023] In a 1000ml three-necked flask, add 250g of water, 100g of o-nitrotoluene, 2g of PEG6002g, 110g of HBr48%, 10g of azobisisobutyronitrile, and dropwise add 80g of 30% hydrogen peroxide at 78-82°C (the above are all in mass ratio) , After about 2 hours of dripping, keep warm at this temperature for 2 hours until the red color fades away, then remove the upper water layer, wash the lower layer with 5% sodium sulfite, and then wash with water, add 100m1 petroleum ether, stir and heat up Dissolved; after dissolving, the petroleum ether solution was lowered to room temperature, and the solution was layered. Petroleum ether was rinsed to obtain o-white nitrobenzyl bromide solid with a content of 78.5% and a yield of 79.4%.
Embodiment 3
[0025] In a 1000ml three-neck flask, add 250g of water, 100g of o-nitrotoluene, 2g of PEG6002g, 110g of HBr48%, 4g of azobisisobutyronitrile, and dropwise add 50g of 30% hydrogen peroxide at 78-82°C, then cool down to 55°C, Add 6g of azobisisobutyronitrile, continue to add 30g of 30% hydrogen peroxide dropwise at this temperature (the above are all mass ratios), after the addition is completed, keep the reaction at this temperature for 6 hours until the red color fades away, and then remove the upper layer The water layer, the lower material layer was washed with 5% sodium sulfite, and then washed with water, and 100ml of petroleum ether was added, stirred and heated to dissolve; after dissolution, the petroleum ether solution was lowered to room temperature, and the solution was layered. The lower layer was the raw material of o-nitrotoluene, Put the upper layer of petroleum ether solution directly into the refrigerator to refrigerate and let it stand, white crystals precipita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com