Docetaxel liposome and preparation method thereof
A technology of docetaxel and liposomes, which is applied in the field of medicine, can solve the problems of difficult industrial production, easy oxidation of liquid liposomes, and low drug loading of liposomes, and achieve easy industrial production and short production time , the effect of controllable drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Dissolve 1g of docetaxel, 25g of soybean lecithin and 3g of cholesterol in 800ml of tert-butanol, add 1% activated carbon to filter out the pyrogen;
[0044] 2) At 40°C, add 50g of sodium chloride carrier powder, and remove the organic solvent under reduced pressure to form a dry powder;
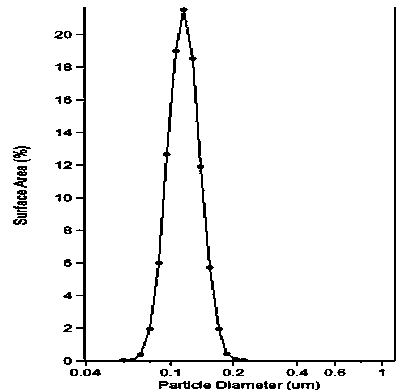
[0045] 3) At 40°C, add 1000ml of hydration medium containing phosphate buffer (PH6.5), reduce the particle size of the hydration solution by external force (probe ultrasound), and filter and sterilize through a 0.22μm filter membrane. Obtain a uniform liposome solution with blue opalescence;
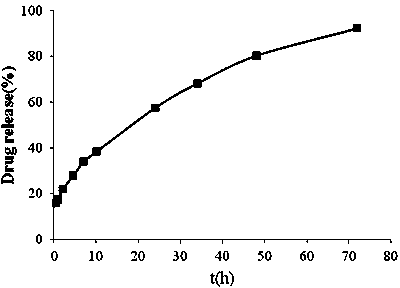
[0046] 4) Add 50g of lactose, remove the water after freeze-drying, and obtain dry docetaxel liposome freeze-dried powder injection, reconstitute into liposome after hydration and shaking before use, and inject it.
[0047] The concentration of docetaxel in the prepared liposome was 1 mg / ml, and the encapsulation efficiency was 93.3%.
Embodiment 2
[0049] 1) Dissolve 5g of docetaxel, 150g of sphingomyelin and 75g of cholesterol in 1000ml of ethanol, add 1% activated carbon to filter out the pyrogen;
[0050] 2) At 45°C, add 200g of sorbitol carrier powder, and remove the organic solvent under reduced pressure to form a dry powder;
[0051] 3) At 45°C, add 1000ml of hydration medium (PH7.4) containing acetate buffer, reduce the particle size of the hydration solution by external force (ultrasonic oscillation), and filter and sterilize through a 0.22μm filter membrane , to obtain a uniform liposome solution with blue opalescence;
[0052] 4) Add 150g of dextran, remove the water after lyophilization, and obtain dry docetaxel liposome lyophilized powder injection, reconstitute into liposome after hydration and shaking before use, and inject it.
[0053] The concentration of docetaxel in the prepared liposome was 5 mg / ml, and the encapsulation efficiency was 89.5%.
Embodiment 3
[0055] 1) Dissolve 2g of docetaxel, 10g of cardiolipin and 2.5g of cholesterol in 600ml of chloroform, add 1% activated carbon and filter to remove pyrogens;
[0056] 2) At 50°C, add 100g of sucrose carrier powder, remove the organic solvent under reduced pressure, and form a dry powder;
[0057] 3) At 50°C, add 1000ml of hydration medium containing carbonate buffer (PH7.4), reduce the particle size of the hydrated solution by external force (high pressure homogenization), and filter through a 0.22μm filter membrane to remove bacteria, to obtain a uniform liposome solution with blue opalescence;
[0058] 4) Add 50g of sorbitol, remove the water after freeze-drying, and obtain dry docetaxel liposome freeze-dried powder injection, reconstitute into liposome after hydration and shaking before use, and inject it.
[0059] The concentration of docetaxel in the prepared liposome was 2 mg / ml, and the encapsulation efficiency was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com