Dilute sulfuric acid diazotization process of substituted phenylamine
A technology of diazotization and dilute sulfuric acid, applied in azo dyes, organic chemistry, monoazo dyes, etc., can solve the disadvantages of cost reduction and environmental protection, difficulty in controlling coupling temperature, and increased consumption of sulfuric acid and other problems, to achieve the beneficial effects of reducing the amount of freezing and ice usage, increasing equipment, and reducing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
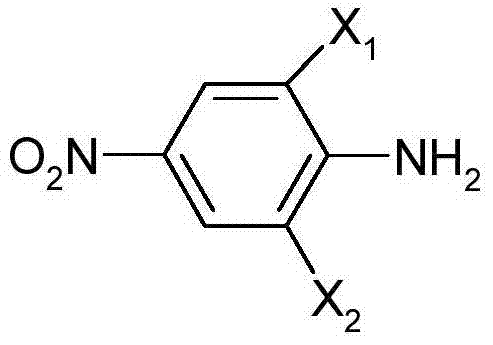
[0018] Add 20g of 65% sulfuric acid and 39g of 40% nitrosylsulfuric acid solution into a 100ml flask, and add 25g of 6-chloro-2,4-dinitroaniline with stirring at a temperature of 25-30°C. The temperature was kept at ℃ for 3 hours, and it was set aside for coupling.
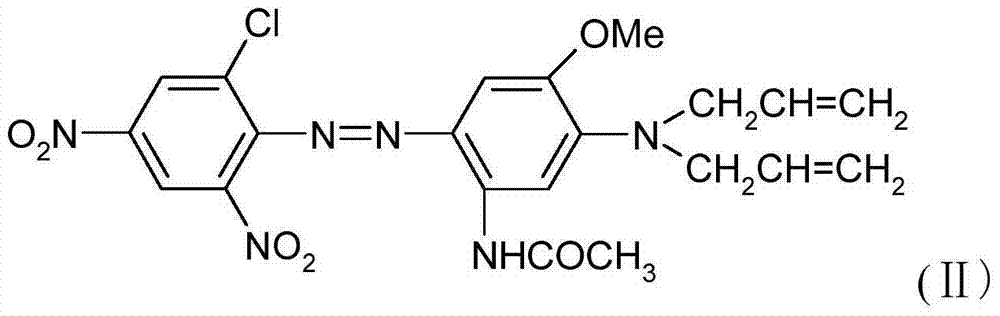
[0019] In a 2000ml beaker, add 1000g of ice water, 0.5g of OP, 2g of sulfamic acid, 30g of N,N-diallyl-2-methoxy-5-acetamidoaniline, stir for 30 minutes, then add the above diazo solution dropwise Coupling is carried out, and then the disperse blue dye of formula (II) is obtained by filtering and washing with water, and the dye yield is 95%.
[0020]
Embodiment 2
[0022] Add 20g of 95% sulfuric acid and 39g of 40% nitrosylsulfuric acid solution into a 100ml flask, and add 25g of 6-chloro-2,4-dinitroaniline with stirring at a temperature of 25-30°C. The temperature was kept at ℃ for 3 hours, and it was set aside for coupling.
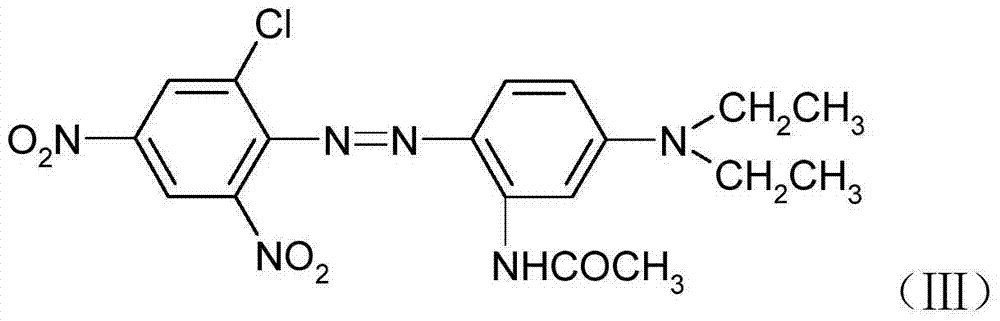
[0023] In a 2000ml beaker, add 1000g of ice water, 0.4g of OP, 2g of sulfamic acid, 24g of N,N-diethyl-3-acetamidoaniline, stir for 30 minutes, add the above diazo solution dropwise for coupling, then filter and wash with water to obtain Formula (Ⅲ) disperse purple dye, dye yield 94%.
[0024]
Embodiment 3
[0026] Add 24g of 40% sulfuric acid and 30g of 40% nitrosylsulfuric acid solution into a 100ml flask, and add 20g of 2,6-dichloro-4-nitroaniline with stirring at a temperature of 25 to 30°C. The temperature was kept at ℃ for 3 hours, and it was set aside for coupling.
[0027] In a 2000ml beaker, add 1000g ice water, 0.4g Pingping O, 2g sulfamic acid, 23g N-cyanoethyl-N-acetoxyethylaniline, after stirring for 30 minutes, drop the above diazo solution for coupling, and then The disperse purple dye of formula (IV) was obtained by filtering and washing with water, and the dye yield was 94.5%.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com