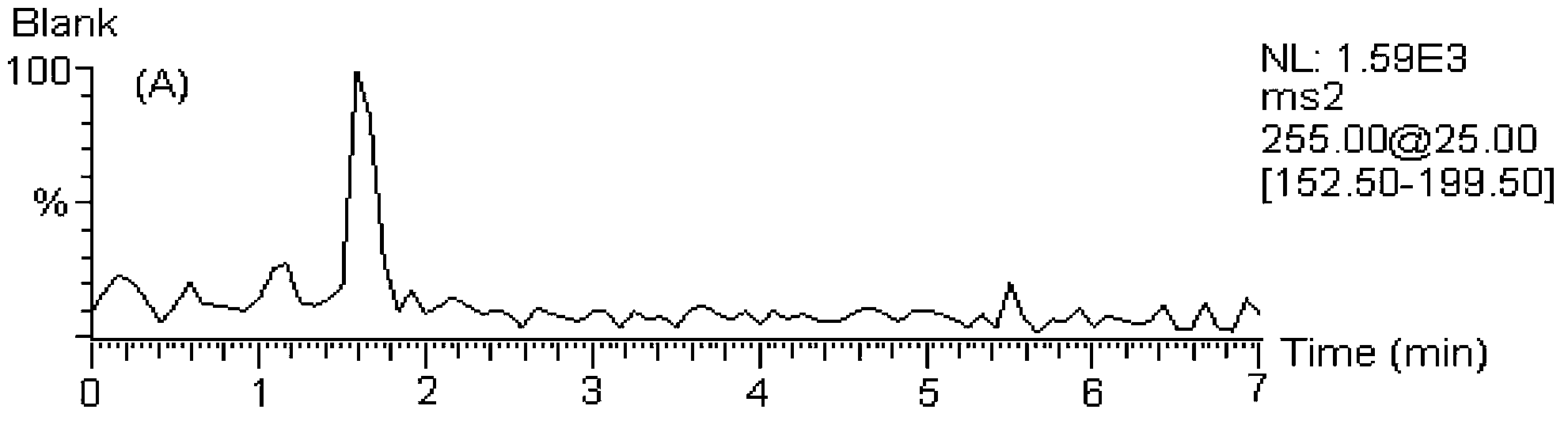
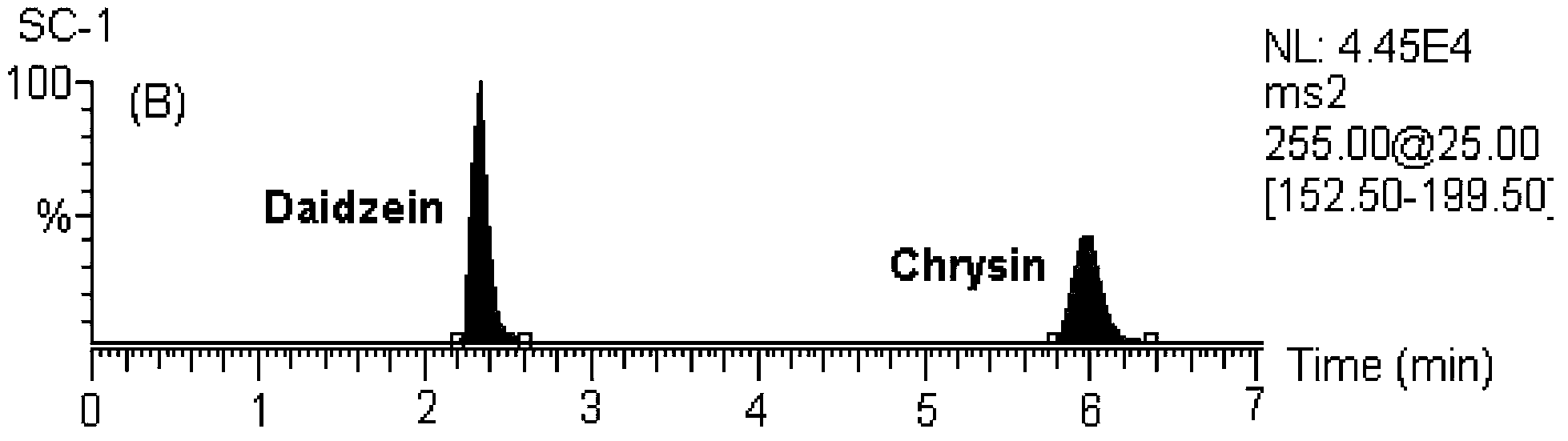
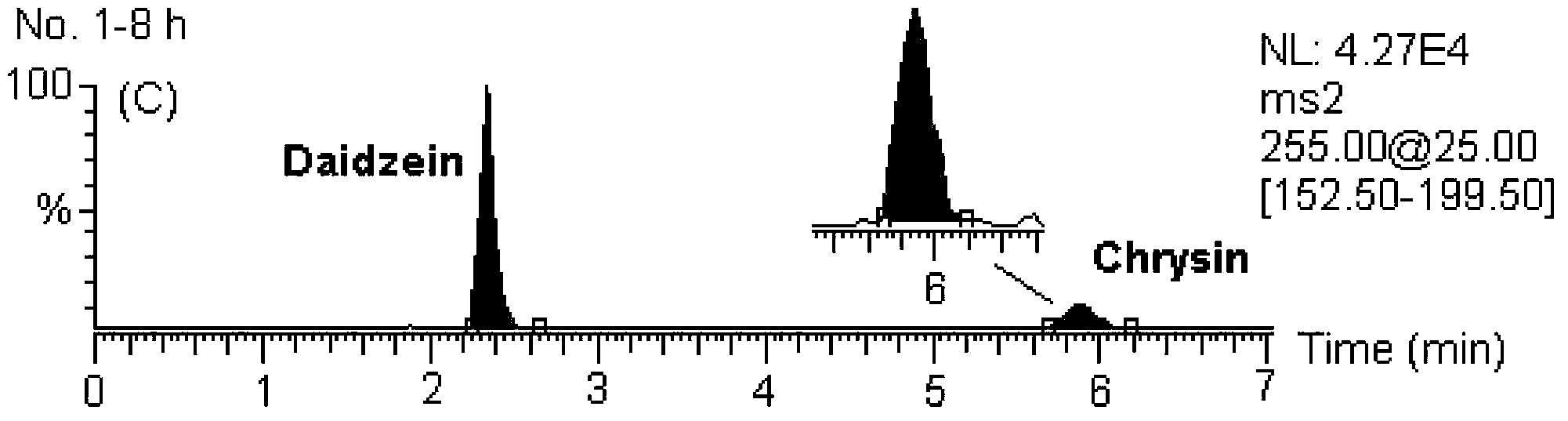
Detecting method for 5 flavones and 4 alkaloids in blood plasma
A technique of plasma and legal quantification, applied in the fields of chemistry and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] LC-MS / MS conditions
[0139] Column: ZORBAX SB-C 18 (150×2.1mm, 5μm, Agilent)
[0140] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d, 5μm, Phenomenex)
[0141] Mobile phase: water (containing 0.1% formic acid)-acetonitrile (30:70, v / v)
[0142] Column temperature: 25°C
[0143] Flow rate: 200μL·min -1
[0144] Ion source: electrospray ionization source (ESI)
[0145] Ionization mode: positive ion mode
[0146] MS / MS: Selected Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z 255→153 (chrysin), m / z 285→270 (wogonin and scutellarin A), m / z 273→153 (naringenin), m / z z 303→153 (hesperetin), m / z 255→199 (internal standard, daidzein), m / z 320→292 (coptisine), m / z 338→322 (drugine), m / z z352→336 (palmatine), m / z 336→320 (berberine), m / z 356→192 (internal standard, tetrahydropalmatine).
[0147] Mass spectrometer parameters: spray voltage: 4000V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (35psi); Au...
Embodiment 2
[0156] LC-MS / MS conditions
[0157] Column: XBridge-C 18 (150×2.1mm, 5μm, Waters)
[0158] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d, 5μm, Agilent)
[0159] Mobile phase: water (containing 0.01% formic acid)-acetonitrile (60:40, v / v)
[0160] Column temperature: 25°C
[0161] Flow rate: 200μL·min -1
[0162] Ion source: electrospray ionization source (ESI)
[0163] Ionization mode: positive ion mode
[0164]MS / MS: Selected Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z 255→153 (chrysin), m / z 285→270 (wogonin and scutellarin A), m / z 273→153 (naringenin), m / z z 303→153 (hesperetin), m / z 255→199 (internal standard, daidzein), m / z 320→292 (coptisine), m / z 338→322 (drugine), m / z z352→336 (palmatine), m / z 336→320 (berberine), m / z 356→192 (internal standard, tetrahydropalmatine).
[0165] Mass spectrometer parameters: spray voltage: 4000V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (35psi); Auxiliar...
Embodiment 3
[0172] LC-MS / MS conditions
[0173] Column: WondaSil-C 18 (150×2.1mm, 5μm, Shimadzu)
[0174] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d, 5μm, Phenomenex)
[0175] Mobile phase: water (containing 0.5% formic acid)-acetonitrile (30:70, v / v)
[0176] Column temperature: 25°C
[0177] Flow rate: 200μL·min -1
[0178] Ion source: electrospray ionization source (ESI)
[0179] Ionization mode: positive ion mode
[0180] MS / MS: Selected Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z 255→153 (chrysin), m / z 285→270 (wogonin and scutellarin A), m / z 273→153 (naringenin), m / z z 303→153 (hesperetin), m / z 255→199 (internal standard, daidzein), m / z 320→292 (coptisine), m / z 338→322 (drugine), m / z z352→336 (palmatine), m / z 336→320 (berberine), m / z 356→192 (internal standard, tetrahydropalmatine).
[0181] Mass spectrometer parameters: spray voltage: 4000V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (35psi); Au...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com