Continuous reaction technological method for preparing epsilon-caprolactone, and microchannel reaction equipment
A technology of microchannel reaction and process method, applied in the field of organic synthesis process, to reduce the generation of by-products, improve reaction efficiency, and improve safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
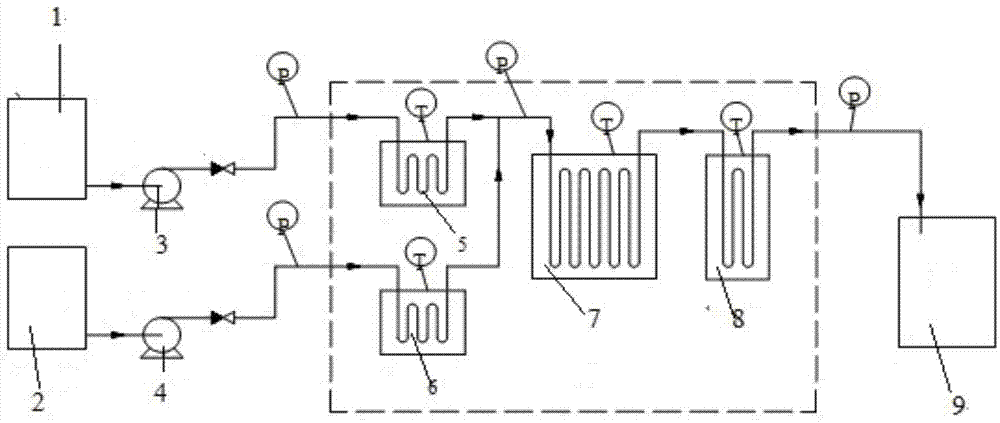
[0031] (1) Device: Microchannel reaction device The microchannel adopts ordinary round pipes ( image 3 -a), the material is PFA, and the channel feature size is 2.0mm. refer to figure 1 Shown technological process of the present invention, set up as figure 2 microchannel reaction device.
[0032] (2) Preparation of peroxyacid: Set the mass flow rate ratio of metering pump A and metering pump B to acetic anhydride: 30% hydrogen peroxide = 1.0:1. After the two materials are fully preheated, the The mixed reaction is carried out in the reaction zone, the reaction temperature is set at 20°C, the residence time of the reaction material is controlled by adjusting the flow rate of the metering pump and the length of the microchannel to be 10s, and the material is collected from the outlet of the microchannel reaction system to obtain The mass fraction is 15.5% peracetic acid.
[0033] (3) Preparation of ε-caprolactone: Adjust the configuration scheme of the preheating zone, rea...
Embodiment 2
[0035] (1) Device: Microchannel reaction device The microchannel adopts a rectangular flat pipe ( image 3 -b), the material is stainless steel, the characteristic dimensions of the channel are a=2.0mm, b=2.0mm (a is the channel flow width, b is the channel flow thickness). refer to figure 1 Shown technological process of the present invention, set up as figure 2 microchannel reaction device.
[0036] (2) Preparation of peroxyacid: Set the mass flow rate ratio of metering pump A and metering pump B to acetic anhydride: 50% hydrogen peroxide = 1.2:1. After the two materials are fully preheated, the The mixed reaction is carried out in the reaction zone, the reaction temperature is set at 40°C, the residence time of the reaction material is controlled by adjusting the flow rate of the pump to be 100s, and the material is collected from the outlet of the microchannel reaction system, which is the process with a mass fraction of 18.7%. Oxyacetic acid.
[0037] (3) Preparation...
Embodiment 3
[0039] (1) Device: Microchannel reaction device The microchannel adopts a pulsed circular pipe microchannel with a built-in throttling orifice ( image 3 -c shown), the material is glass, the channel characteristic dimension Ф 1 =3.0mm, Ф 2 =1.0mm, (Ф 1 is the flow diameter of the main channel of the circular pipe, Ф 2 is the flow diameter of the flow-limiting orifice of the throttle orifice in the circular pipe). refer to figure 1 Shown technological process of the present invention, set up as figure 2 microchannel reaction device.
[0040] (2) Preparation of peroxyacid: Set the mass flow rate ratio of metering pump A and metering pump B to acetic anhydride: 50% hydrogen peroxide = 1.5:1. The mixed reaction is carried out in the reaction zone, the reaction temperature is set at 40°C, the residence time of the reaction material is controlled by adjusting the flow rate of the metering pump and the length of the microchannel to be 180s, and the material is collected from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com