Polymer single-ion electrolyte and preparation method thereof
A polymer and electrolyte technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of low room temperature conductivity, poor mechanical strength and film-forming performance, cumbersome synthesis steps, etc., and achieve high room temperature conductivity, mechanical Good strength and film-forming performance, high lithium ion transfer number effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
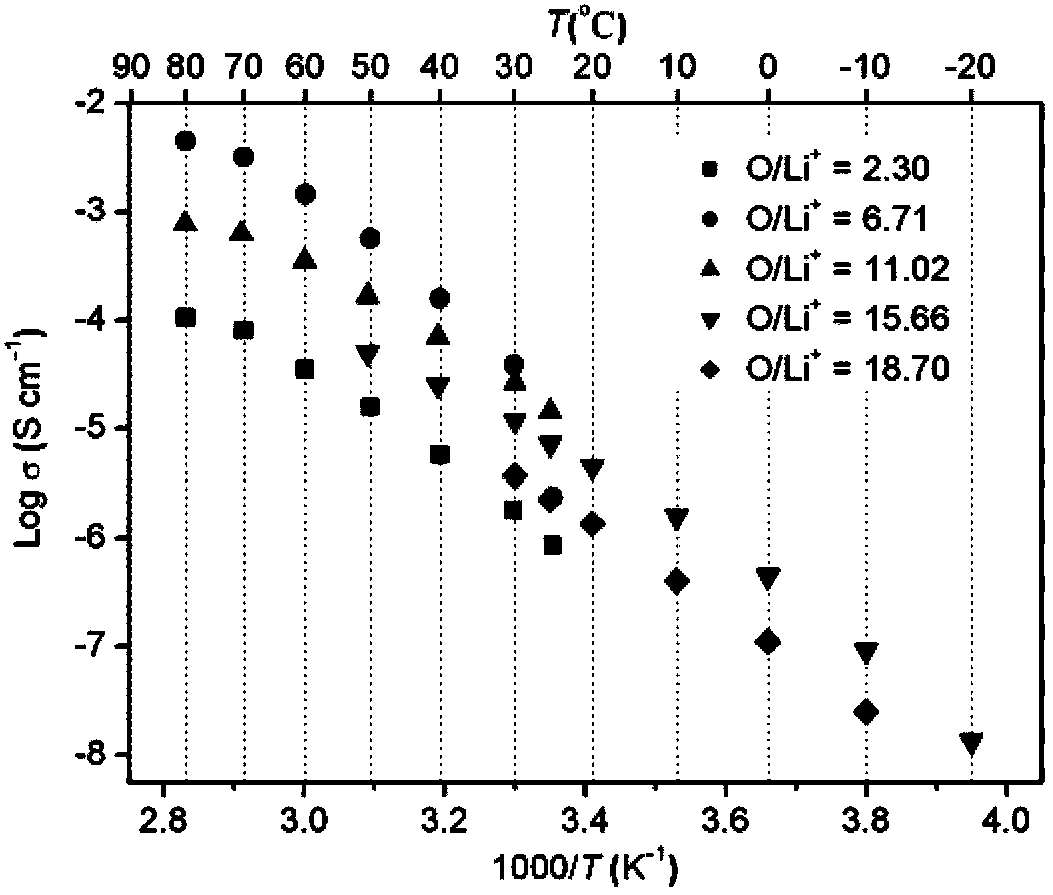
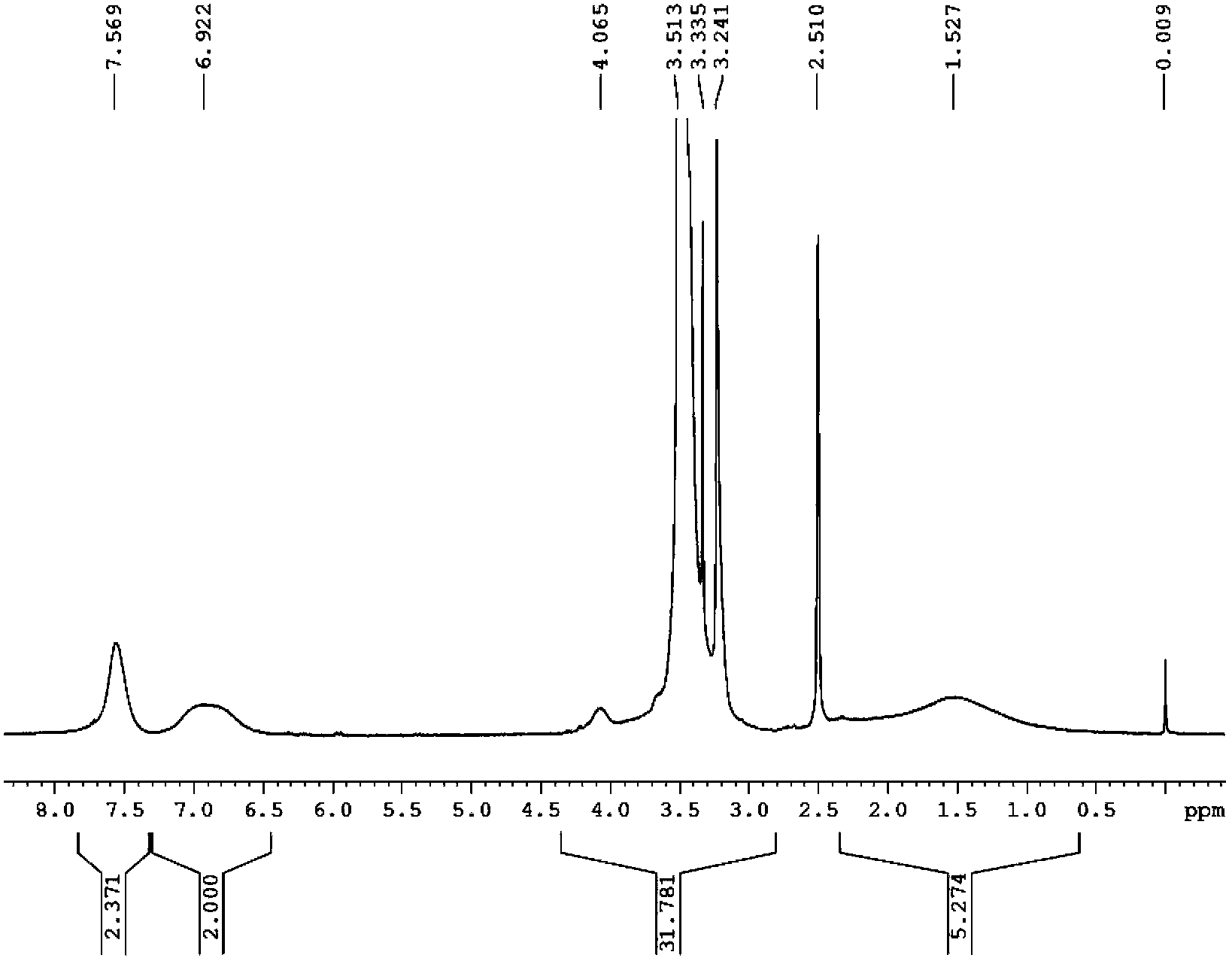
Image
Examples
Embodiment 1-10
[0032] Embodiment 1-10 is the preparation of polymer single ion electrolyte
Embodiment 1
[0033] Example 1: Preparation of (p-vinylbenzenesulfonyl) (fluorosulfonyl) lithium imide (LiSFSI) and methoxytriethylene glycol acrylate (MPEGA, n=3) copolymer (electrolyte 1)
[0034] Add 1.32g (5.0mmol) (p-vinylbenzenesulfonyl)(fluorosulfonyl)imide lithium (LiSFSI) monomer, 1.09g (5.0mmol) methoxytriethylene glycol acrylic acid to a 25mL reaction flask Ester (MPEGA, n=3), 0.0042 g (0.025 mmol) azobisisobutyronitrile (AIBN) and 2.5 mL dry DMF. Pass argon to exhaust oxygen for 2h, and react at 50°C for 8h. After the reaction was completed, cool to room temperature, and slowly drop the reaction solution into excess diethyl ether under stirring to precipitate a viscous solid, pour out the upper layer of diethyl ether slowly, and repeat the dissolution and precipitation three times. A gel-like polymer solid was obtained; the viscous polymer was vacuum-dried at 80° C. for 8 hours to obtain 1.6 g of random copolymer (electrolyte 1). Through NMR characterization, the actual oxyeth...
Embodiment 2
[0036] Embodiment 2: (p-vinylbenzenesulfonyl) (trifluoromethylsulfonyl) lithium imide (LiSTFSI) and methoxy octaethylene glycol acrylate (MPEGA, n=8) copolymer (electrolyte 2) preparation
[0037] Add 1.32g (4.1mmol) (p-vinylbenzenesulfonyl)(trifluoromethylsulfonyl)imide lithium (LiSTFSI) monomer, 1.80g (3.7mmol) methoxy octaacetal to a 25mL reaction flask Diol (400) acrylate (MPEGA, n=8), 0.013 g (0.08 mmol) azobisisobutyronitrile (AIBN) and 5 mL dry DMF. Pass argon to exhaust oxygen for 2h, and react at 60°C for 15h. After the reaction was completed, cool to room temperature, and slowly drop the reaction solution into excess diethyl ether under stirring to precipitate a viscous solid, pour out the upper layer of diethyl ether slowly, and repeat the dissolution and precipitation three times. A gel-like polymer solid was obtained; the viscous polymer was vacuum-dried at 80° C. for 8 hours to obtain 42 g of a random copolymer (electrolyte 2).
[0038] Dissolve 42 g of the ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com