2, 4-pyrrolidine-diketone compounds and synthesis method thereof
A compound and diketone technology, applied in the field of new compounds and their synthesis, can solve the problems of poor stereoselectivity, long route, low total yield, etc., and achieve the effects of good biological activity, short route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
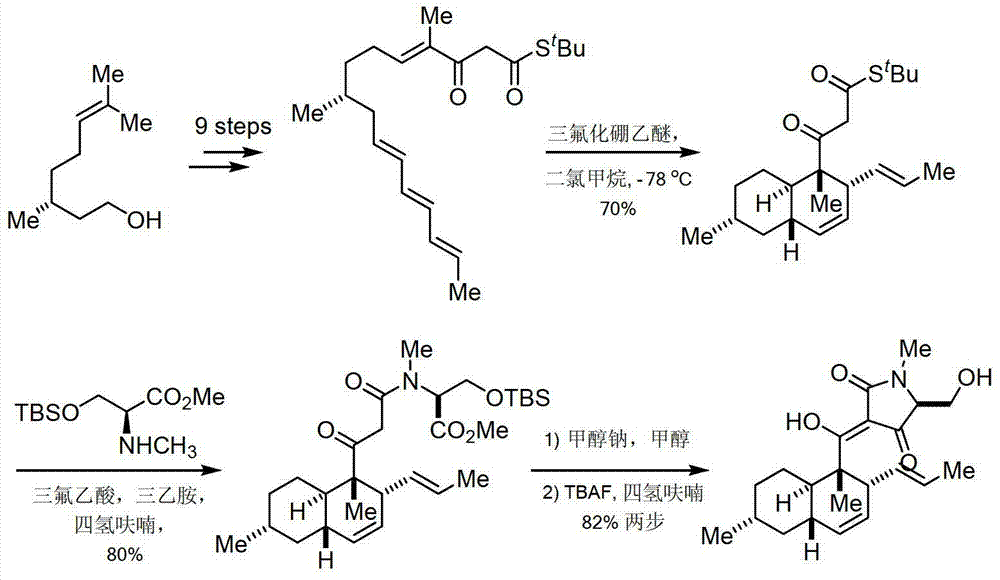
Image
Examples
Embodiment 1
[0047] Synthesis of compound 5
[0048]
[0049] Synthesis of compound 11: Weigh 10 (3.4g, 8.70mmol) in a 200mL dry reaction flask, add 44mL (0.2M) of tetrahydrofuran, add n-butyllithium (6mL, 9.6mmol) dropwise at -78°C, and After reacting at ℃ for 10 min, add 9 (872 mg, 2.90 mmol) in 5 mL THF at -78 °C, react at this temperature for 30 min, then raise to 0 °C and react for 10 min. After the reaction was complete, 20 mL of saturated ammonium chloride solution was added thereto, stirred at room temperature for 10 min, the tetrahydrofuran was spin-dried, extracted with (50 mL×2) ethyl acetate, the organic phases were combined and washed with saturated ammonium chloride (20 mL×2) The solution was washed with saturated sodium chloride (20mL×1), dried over anhydrous sodium sulfate, and spin-dried. Flash column chromatography (1% ethyl acetate / petroleum ether) yielded 812 mg of yellow liquid, and the yield was 83%. HRMS(EI):Exact mass calcd for C 20 h 32 SO 2 [M] + :336.212...
Embodiment 2
[0057] Synthesis of compound 6
[0058]
[0059] Synthesis of compound 16: Weigh 15 (147mg, 0.41mmol) in a 50mL reaction flask, add 20mL (0.02M) of tetrahydrofuran and 13 (105mg, 0.82mmol), triethylamine (228μL, 1.64mmol) and incubate at 0°C Quickly added silver trifluoroacetate (142mg, 0.62mmol), reacted at this temperature for 10min. After the reaction was complete, 0.5 mL of saturated sodium chloride solution was added thereto, stirred at room temperature for 5 min, and passed over diatomaceous earth. Purified by column chromatography (30% ethyl acetate / petroleum ether) to obtain 150 mg of an orange solid with a yield of 90%. HRMS(EI):Exact mass calcd for C 24 h 35 NO 4 [M] + :401.2566;Found:401.2567, 1 H NMR (400MHz, CDCl 3)δ5.47–5.27(m,3H),5.26–5.11(m,1H),4.52(dd,J=8.3,3.6Hz,1H),3.77–3.55(m,3H),3.49(t,J= 6.4Hz, 2H), 3.31(d, J=15.8Hz, 1H), 2.54(dd, J=9.1, 4.8Hz, 1H), 2.36(d, J=7.8Hz, 2H), 2.27–2.09(m, 3H),2.09–1.83(m,3H),1.84–1.62(m,3H),1.59(dd,J=8.7,3.6Hz,2H),...
Embodiment 3
[0063] Synthesis of compound 7
[0064]
[0065] Synthesis of compound 18: Weigh 15 (60mg, 0.17mmol) in a 20mL dry reaction tube, add 4mL (0.1M) of tetrahydrofuran and 17 (35mg, 0.24mmol), triethylamine (100μL, 0.68mmol) and dissolve in 0 Silver trifluoroacetate (70 mg, 0.26 mmol) was added quickly at ℃, and reacted at this temperature for 10 min. After the reaction was complete, 0.5 mL of saturated sodium chloride solution was added thereto, stirred at room temperature for 5 min, and passed over diatomaceous earth. Purified by column chromatography (5% ethyl acetate / petroleum ether) to obtain 52 mg of white solid. 75% yield. HRMS(EI):Exact mass calcd for C 25 h 39 NO 4 [M] + :417.2879;Found:417.2877, 1 H NMR (400MHz, CDCl 3 ) 1 H NMR (400MHz, CDCl 3 )δ5.48–5.28(m,3H),5.19(dd,J=15.5,7.6Hz,1H),4.92(t,J=8.8Hz,1H),3.87–3.56(m,4H),3.53–3.30 (m,1H),3.02–2.71(m,3H),2.54(dd,J=8.7,4.7Hz,1H),2.28–2.07(m,1H),1.70(ddd,J=22.5,18.8,9.6Hz ,6H),1.60–1.53(m,3H),1.52–1.37(m,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com