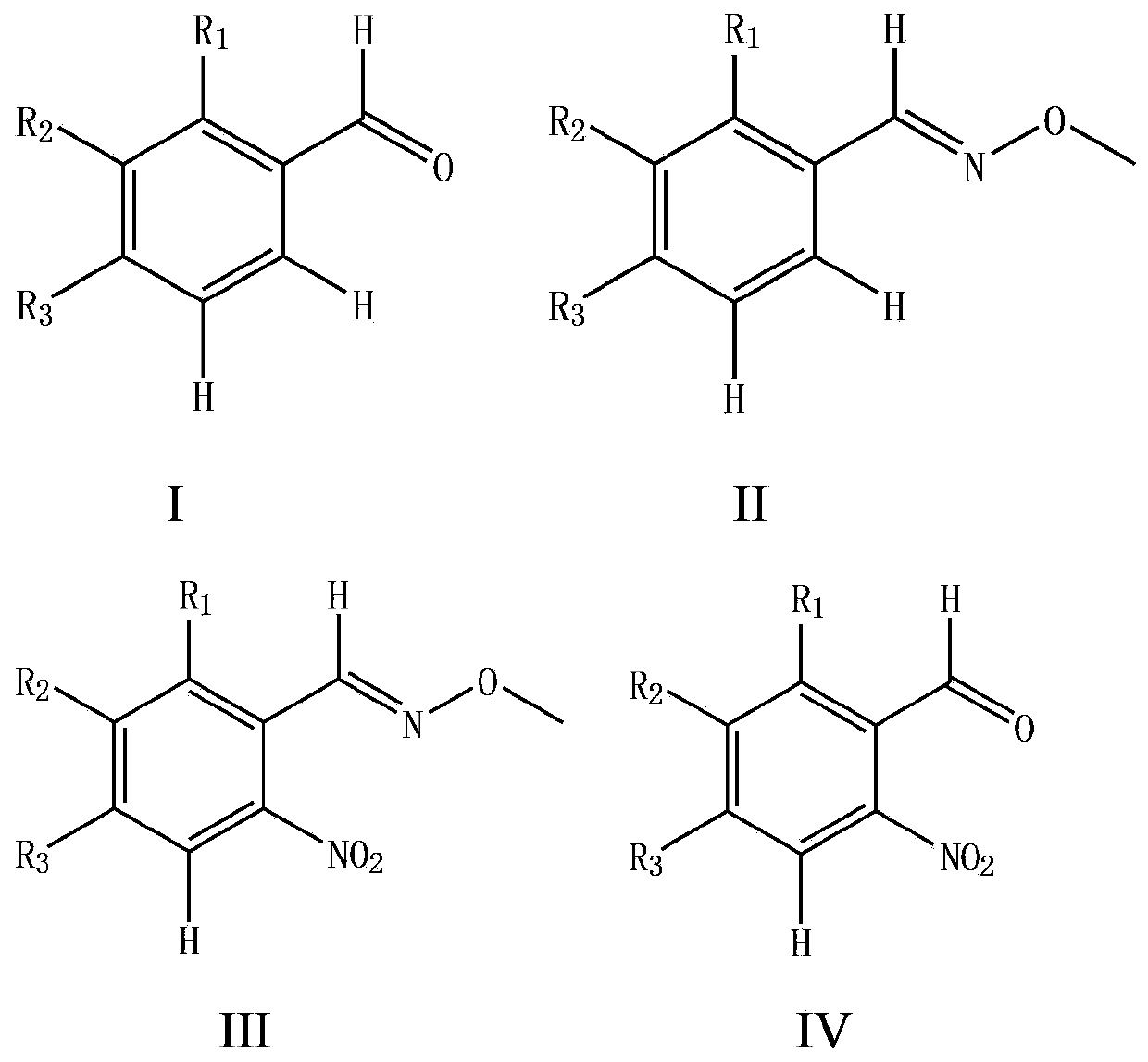
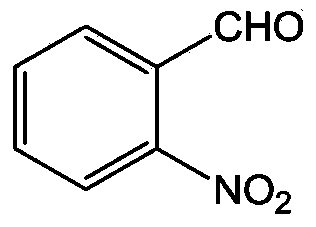
Synthesizing method for o-nitrobenzaldehyde compound
A technology of o-nitrobenzaldehyde and compounds, which is applied in the field of synthesis of organic compounds, can solve the problems of long reaction steps, different reaction steps, difficult region-specific synthesis, etc., and achieves good selectivity, simple reaction steps, low-end good adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Using benzaldehyde as raw material to synthesize 2-nitrobenzaldehyde
[0046]
[0047] (1) Add 0.530g (5.0mmol) of benzaldehyde, 1.12g (13.4mmol) of methoxyamine hydrochloride, 1.804g (22mmol) of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, the reaction was completed after 3 hours, the resulting mixed solution was extracted with 3×45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow liquid benzaldehyde-O-methyl Oxime 0.641g (95% yield).
[0048] (2) Add 67.5 mg (0.5 mmol) of benzaldehyde O-methyloxime, 11 mg (0.05 mmol) of palladium diacetate, 154 mg (1.0 mmol) of silver nitrite, 270 mg (1.0 mmol) of potassium persulfate and 1,2-bis Ethyl chloride (5ml) was successively added to a 10ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 110°C, followed by TL...
Embodiment 2
[0052] Synthesis of 4-trifluoromethyl-2-nitrobenzaldehyde from 4-trifluoromethylbenzaldehyde
[0053]
[0054] (1) Add 0.870g (5.0mmol) of 4-trifluoromethylbenzaldehyde, 1.12g of methoxyamine hydrochloride, 1.804g of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, and the reaction was completed after 3 hours. The resulting mixed solution was extracted with 3 × 45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow liquid 4-trifluoromethylbenzene Formaldehyde-O-methyloxime 0.995g (98% yield).
[0055] (2) 101.5mg (0.5mmol) of 4-trifluoromethylbenzaldehyde-O-methyloxime, 11mg (0.05mmol) of palladium diacetate, 154mg (1.0mmol) of silver nitrite, 270mg (1.0mmol) of potassium persulfate ) and 1,2-dichloroethane (5ml) were sequentially added to a 10ml sealed pressure vessel. The mixture was heated ...
Embodiment 3
[0059] Synthesis of 5-bromo-2-nitrobenzaldehyde from 3-bromobenzaldehyde
[0060]
[0061] (1) Add 0.915g (5.0mmol) of 3-bromobenzaldehyde, 1.12g of methoxyamine hydrochloride, 1.804g of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, the reaction was completed after 3 hours, the resulting mixed solution was extracted with 3 × 45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow solid 3-bromobenzaldehyde-O - Methyloxime 0.994 g (98% yield).
[0062] (2) 101.5mg (0.5mmol) of 3-bromobenzaldehyde-O-methyloxime, 11mg (0.05mmol) of palladium diacetate, 154mg (1.0mmol) of silver nitrite, 270mg (1.0mmol) of potassium persulfate and 1 , 2-dichloroethane (5ml) was sequentially added to a 10ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 110°C, followed by TLC detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com