Plant extract with anti-tumor activity and active ingredients thereof
A technology for extracts and compounds is applied in the field of plant extracts with anti-tumor activity and their active ingredients, and achieves the effects of significant anti-tumor activity, novel structure and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of total alkaloids in the leaves of Populus korean:
[0020] Korean poplar leaves (1600g) were cut into pieces, soaked in 0.2% HCl aqueous solution (5L) for 12h, then percolated and extracted with 0.2% HCl aqueous solution to obtain a total of 29L acidic water extract, which was alkalized to pH with 5% NaOH aqueous solution =11, get about 30L of alkaline water, use HPD-100 type macroporous adsorption resin 1.5kg to dynamically absorb the alkaline water, and elute with 95% ethanol after eluting with NaOH aqueous solution with pH=11 for 4 retention volumes 5 retention volumes, 95% ethanol elution partially recovered the solvent to obtain the total alkaloids (18g).
Embodiment 2
[0021] Example 2: In Vitro Antitumor Activity of Total Alkaloids in the Leaves of Populus Koreana (Example 1)
[0022] Take the test cells in the logarithmic growth phase, and use (2-3)×10 4 The density of cells / mL was inoculated in a 96-well culture plate, 100 μL in each well, and after 24 hours of making it adhere to the wall, 100 μL of the compound to be tested diluted to different concentrations with the culture medium was added, and the culture was continued at 37°C for 96 hours. Then add 50 μL MTT solution to each well and incubate at 37°C for 4 hours, discard the supernatant, add 200 μL DMSO to each well, shake at room temperature for 10 minutes, measure the absorbance value of each well at 570 nm on a microplate reader, set A1 (containing 200 μL DMSO) was used as a blank control well, and cisplatin (CP) was used as a positive control. The tumor cell growth inhibition rate was obtained by using the following formula: 1-(absorbance value of drug-dosed well / absorbance va...
Embodiment 3
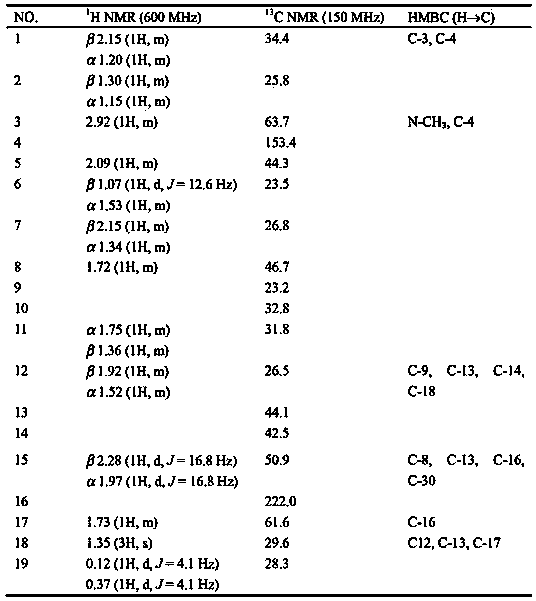
[0026] Example 3: The preparation method of five monomer compounds such as formulas I to V in the total alkaloids of leaves of Populus korean (Example 1)
[0027]18g of the total alkaloid extract of the leaves of Populus korean was used as the raw material, separated by 200-300 mesh silica gel column chromatography, and mixed solvent gradients of petroleum ether-acetone with a volume ratio of 3:1, 1:1 and 0:1 respectively For elution, the eluted part with a volume ratio of petroleum ether-acetone of 3:1 is separated by 200-300 mesh alumina column chromatography, and eluted with a mixed solvent of petroleum ether-acetone with a volume ratio of 100:2 The compound of formula IV (6.0 mg) was obtained, and the compound of formula III (5.2 mg) was obtained by elution with a mixed solvent of petroleum ether-acetone with a volume ratio of 100:10, wherein the volume ratio of petroleum ether-acetone was 1: The eluted part of 1 was then separated by open ODS column chromatography, and el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com