Synthetic method for preparing steroid compounds from 3,17-diketone steroids
A technology of steroid compound and synthesis method, applied in the field of drug synthesis, can solve the problems of not conforming to the concept of green chemistry, not meeting the requirements of green chemistry, restrictions on large-scale industrial production, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
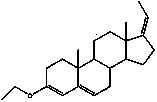
[0059] (1) Step 1: After mixing 1 part of androst-4-ene-3,17-dione (androstenedione, 4AD) with 2-20 parts of ethylene glycol, add 0.01-10% part of p-toluenesulfonic acid Hydrate (TsOH·H 2 O) Catalyst and solvent toluene 50mL, stir, heat reaction in oil bath medium 50-120℃ for 2-10h, stop reaction, cool down; pour into 1:20-50 parts of water, filter, wash solid with water. Purification was recrystallized from methanol. Yield 85%. The reaction was detected by TLC (PE:EtOAc=3:1, color developed by iodine fumigation, Rf=0.72).
[0060]
[0061] C 23 h 34 o 4 , white powdery solid. mp.160-162℃
[0062] =0.80 (petroleum ether (30–60) / EtOAc = 3 / 2).
[0063] 1 H NMR (400 MHz, CDCl 3 ) δ 5.36(s, 1H, H-6), 3.93(m, 8H, 2×OCH 2 CH 2 O), 2.57(d, 1H, J H-H =14Hz, H-9), 2.14-1.10(m, 18H, skeleton protons), 1.04(s, 3H, H-19), 0.87(s, 3H, H-18).
[0064] 13 C NMR (101 MHz, CDCl 3 ) δ 140.14, 121.93, 119.51, 109.44, 65.16, 64.57, 64.46, 64.22, 50.53, 49.50, 45.77, 41.81, 36...
Embodiment 2
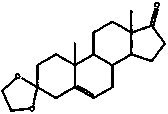
[0108] (1) Step 1: Weigh 1 part of androstenedione and dissolve it in 10-100 parts of THF solution, and keep stirring, add 1-500 parts of absolute ethanol, 1-5 parts of triethyl orthoformate, 0.05-10% p-toluenesulfonic acid, N 2 Protection, the reaction solution is reacted at 25-50°C for 1-12h, after the reaction is complete, the reaction solution is poured into 50-100 parts of water, filtered, and the solid is recrystallized with pyridine / ethanol to obtain a white powdery solid, which is dried and weighed , the yield is about 95%. = 0.73 (petroleum ether(30-60℃) / EtOAc = 3 / 2).
[0109]
[0110] C 21 h 30 o 2 , white powdery solid. mp.144-146℃
[0111] =0.80 (petroleum ether (30–60) / EtOAc = 3 / 2).
[0112] 1 H NMR (400 MHz, CDCl 3 ) δ5.25 (s, 1H, H-6), 5.14(s,1H, H-4), 3.81-3.78 (t, 2H, OCH 2 CH 3 ), 2.52-2.45(m, 1H, H-9), 2.32-1.10(m,16H, skeleton protons), 1.32(t, 3H, OCH 2 CH 3 ), 1.02(s, 3H, H-19), 0.93(s, 3H, H-18).
[0113] 13 C NMR (101 MHz, CDCl 3 )...
Embodiment 3
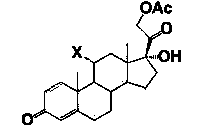
[0146] Using 1,4-androstenedione (ADD) as a raw material, according to the method described in Example 1, a compound with the following structural formula was prepared:
[0147]
[0148] NMR data:
[0149] 1 HNMR (CDCl 3 ): δ=0.94 (3H, H-19), 1.18 (3H, H-18), 2.15 (3H, 21-OCOCH 3 ), 5.24(2H, H-21), 6.12(1H, H-4), 6.35(1H, H-1), 6.69(1H, H-2).
[0150] 13 CNMR (CDCl 3 ): Δ = 90.8, 48.0, 49.6, 33.2, 23.8, 185.7, 35.8, 30.3, 168.1, 124.2, 128.3, 155.4, 43.4, 52.3, 22.8, 32.9, 170.2, 206.8, 16.7, 18.7, 20.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com