Method for analyzing amitraz residual marker
A marker and formamidine technology, which is applied in the field of detection of formamidine residue markers and achieves the effect of high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
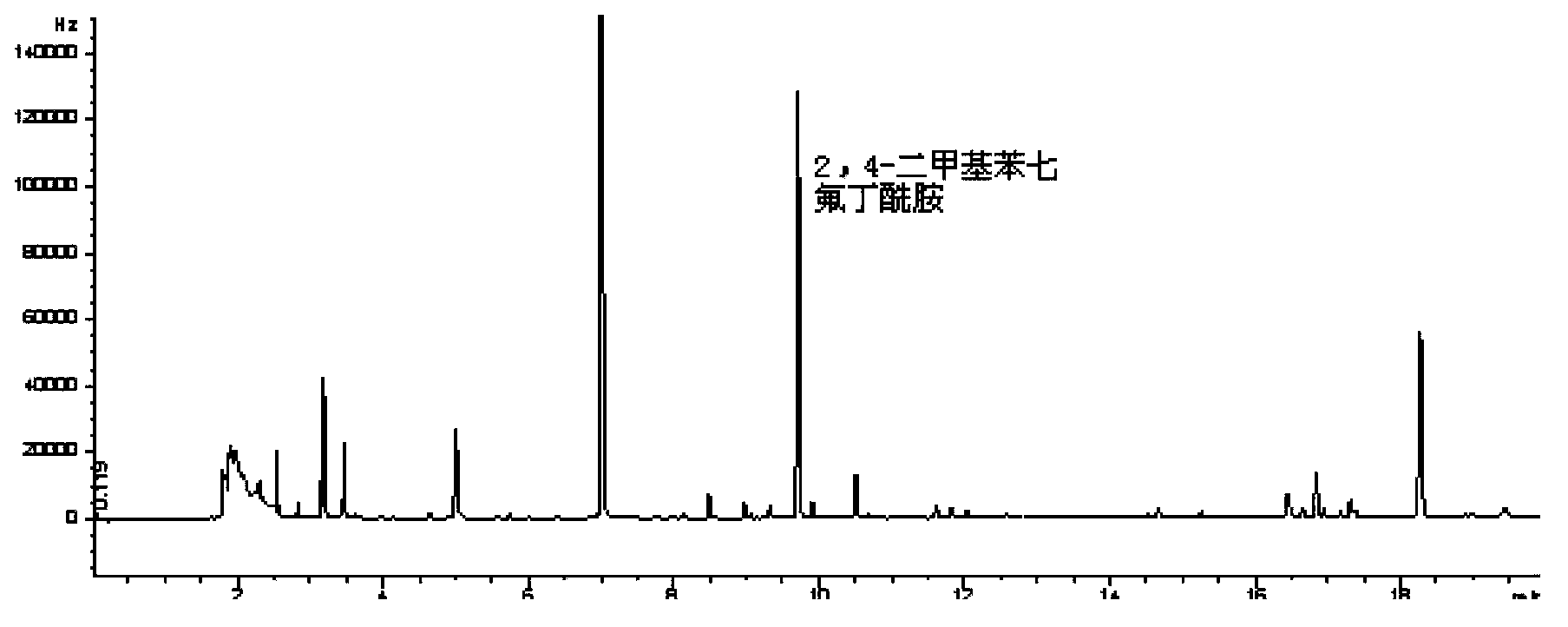
[0034] Example 1: Derivatization reaction of formamidine residue markers with heptafluorobutyric anhydride
[0035] (1) Amitraz and 2,4-dimethylaniline react with heptafluorobutyric anhydride
[0036] Accurately weigh an appropriate amount of amitraz and 2,4-dimethylaniline standard substances, dilute them with n-hexane, and prepare the concentrations of 10 μg / L, 20 μg / L, 50 μg / L, 100 μg / L, 200 μg / L, 400μg / L, 800μg / L mixed standard solution. Measure the mixed standard solution into a polypropylene centrifuge tube, add 10 mL of alkaline aqueous solution (take 1000 mL of distilled water, adjust the pH value to 9.0 with 1 mol / L sodium hydroxide solution), vortex to mix, and centrifuge at 4200 r / min for 10 min. Transfer the supernatant to a polypropylene centrifuge tube, repeat the extraction once, and combine the two extracts. Stand for hydrolysis in a constant temperature drying oven at 70°C for 50 minutes. After cooling, add 5.0 mL of n-hexane to the extract, vortex to mix, ...
Embodiment 2
[0053] Embodiment 2: the mensuration of sample
[0054] (1) Extraction of samples
[0055] The fat, liver, and kidney tissues of pigs, cattle, and sheep were selected as experimental samples, and the residues of amitraz and its metabolite 2,4-dimethylaniline were detected. There are 3 additive concentrations: 20μg / L, 200μg / L, and 400μg / L for fat, liver, and kidney tissues of cattle; 20μg / L, 400μg for fat, liver, and kidney tissues of pigs, cattle, and sheep, respectively. / L and 800μg / L, and set a blank sample respectively. Including three concentrations of limit of quantitation and Chinese meaning (MRL) and 2MRL, each concentration has 5 repeated samples, measured 3 times at different times, and the recovery rate is calculated by the external standard method (reference).
[0056] Weigh (5±0.05) g of the above-listed test materials into a polypropylene centrifuge tube, add 10 mL of alkaline distilled water, vortex and mix, centrifuge at 4200 r / min for 10 min, transfer the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com