Modification method of polyethyleneglycol of protein
A technology of PEGylation and polyethylene glycol, applied in the field of protein modification, can solve the problems of inapplicable functional proteins, limited application scope, inability to carry out PEG modification reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Solid-phase PEG modification of lysozyme based on hydrophobic chromatography column
[0022] Include the following steps:
[0023] (1) Pre-equilibrate the HiTrap Butyl FF column (column volume CV=1mL) with phosphate buffer PB (20mM, pH6.0, 2MNaCl) containing 2M NaCl.
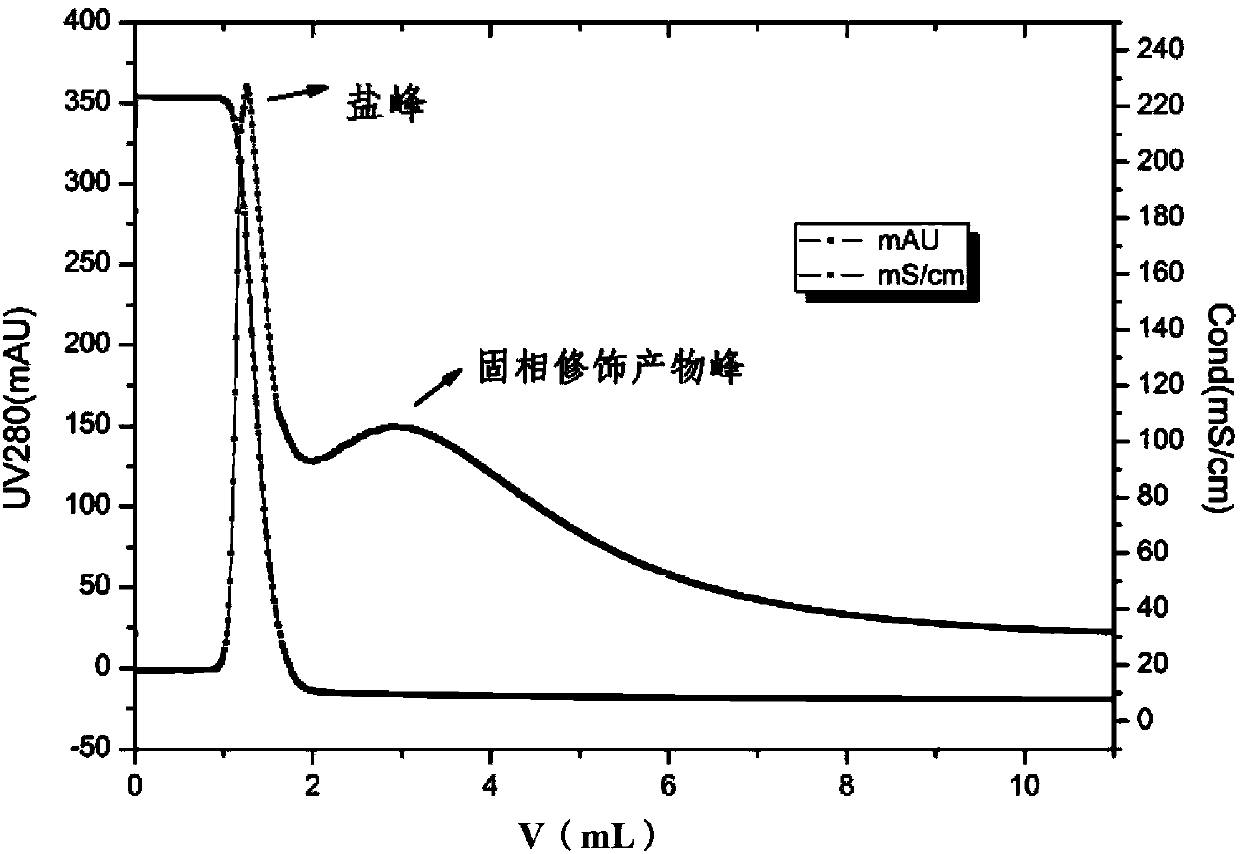
[0024] (2) Use the above PB buffer (20mM, pH6.0, 2M NaCl) to prepare lysozyme solution with a concentration of 1mg / mL lysozyme solution, take 5mL and load it on the HiTrap Butyl FF chromatographic column at a flow rate of 1mL / min. After sample loading, wash with PB buffer (20mM, pH6.0, 2M NaCl) until no protein breaks through.
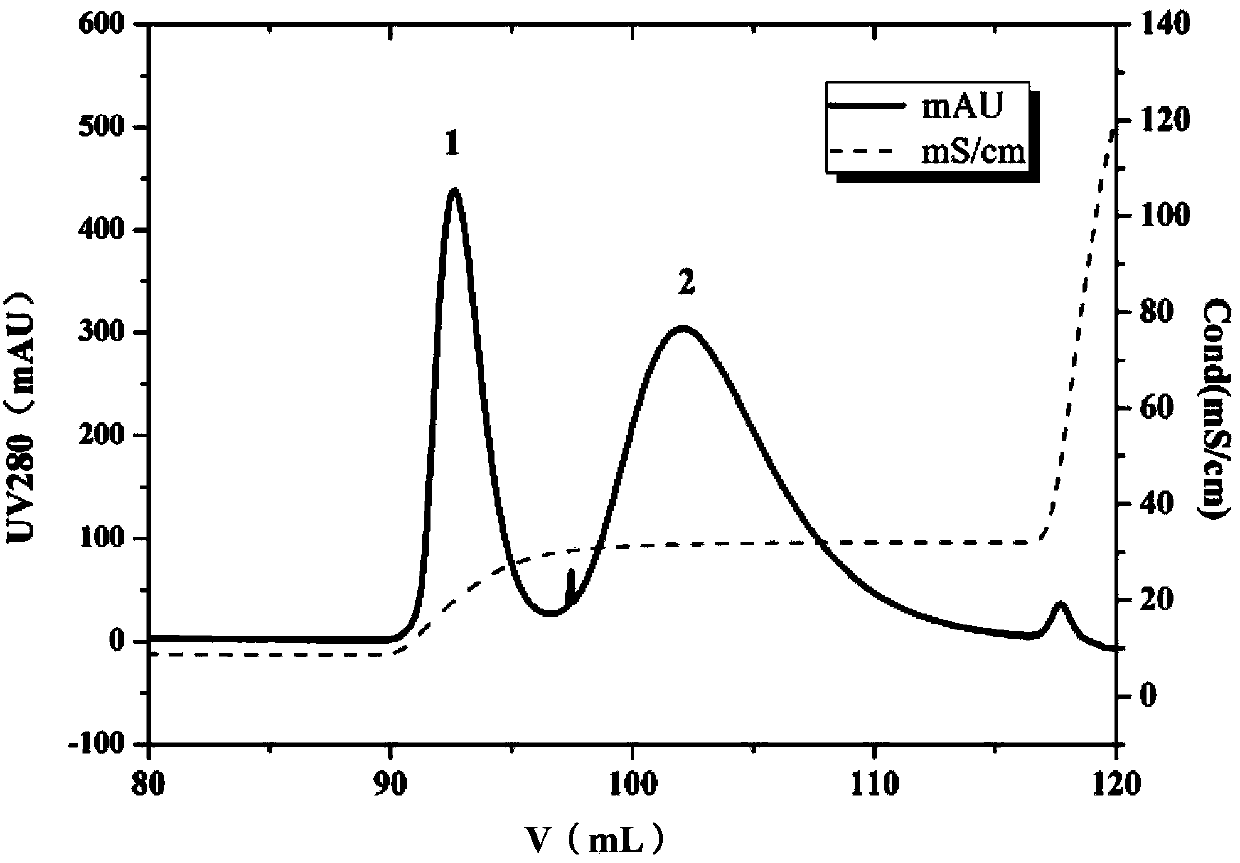
[0025] (3) To contain 30mM NaBH 3 Prepare 10mL of 2mg / mL polyethylene glycol-butyraldehyde (20kDa) solution in PB buffer (20mM, pH6.0, 2MNaCl) of CN, and use the mPEG-butyraldehyde solution as the mobile phase at a flow rate of 1mL / min Pass through the HiTrap Butyl FF chromatographic column, keep the column temperature at room temperature, and wrap the HiTrap Butyl FF...
Embodiment 2
[0030] Example 2 Solid-phase PEG modification of lysozyme based on hydrophobic chromatography column
[0031] Include the following steps:
[0032] (1) Pre-equilibrate the HiTrap Phenyl FF column (column volume CV=1mL) with phosphate buffer PB (20mM, pH6.0, 2MNaCl) containing 2M NaCl.
[0033] (2) Use the above PB buffer (20mM, pH6.0, 2M NaCl) to prepare lysozyme solution with a concentration of 1mg / mL lysozyme solution, take 5mL and load it on the HiTrap Phenyl FF chromatographic column at a flow rate of 1mL / min. After sample loading, wash with PB buffer (20mM, pH6.0, 2M NaCl) until there is no breakthrough.
[0034] (3) To contain 30mM NaBH 3 Prepare 10mL of 2mg / mL polyethylene glycol-butyraldehyde (5kDa) solution in PB buffer (20mM, pH6.0, 2MNaCl) of CN, and use the mPEG-butyraldehyde solution as the mobile phase at a flow rate of 1mL / min Pass through the HiTrap Phenyl FF chromatographic column, keep the column temperature at room temperature, and wrap the HiTrap Phenyl FF...
Embodiment 3
[0039] Example 3 α-chymotrypsin solid-phase PEG modification based on hydrophobic chromatography column
[0040] Include the following steps:
[0041] (1) Pre-equilibrate the HiTrap Phenyl FF column (column volume CV=1mL) with phosphate buffer PB (20mM, pH6.0, 2MNaCl) containing 2M NaCl.
[0042] (2) Use the above PB buffer (20mM, pH6.0, 2M NaCl) to prepare α-chymotrypsin into a protein solution with a concentration of 1mg / mL, take 5mL and load it on a HiTrap Phenyl FF column at a flow rate of 1mL / min, After the sample loading is completed, wash with PB buffer (20mM, pH6.0, 2M NaCl) until there is no breakthrough.
[0043] (3) To contain 30mM NaBH 3 Prepare 10mL of 2mg / mL polyethylene glycol-propionaldehyde (20kDa) solution in CN's PB buffer (20mM, pH6.0, 2MNaCl), and use this solution as the mobile phase to flow through HiTrap Phenyl FF at a flow rate of 1mL / min For chromatographic column, keep the column temperature at room temperature, and wrap the HiTrap Phenyl FF chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com