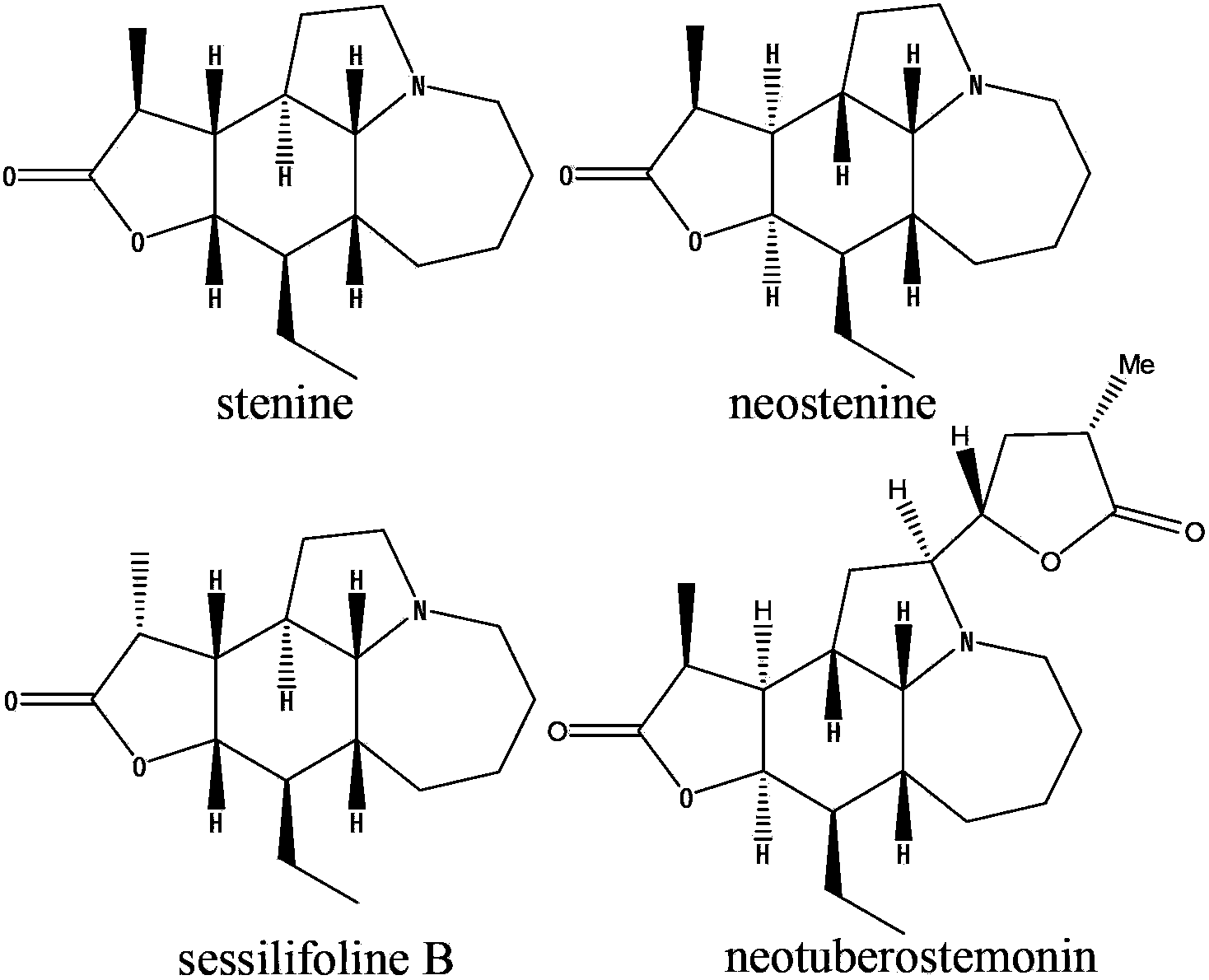
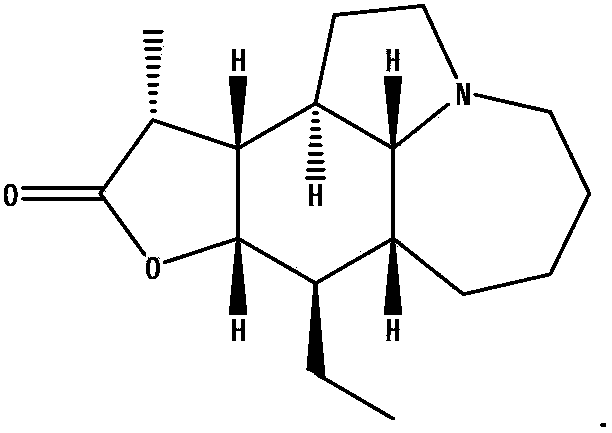
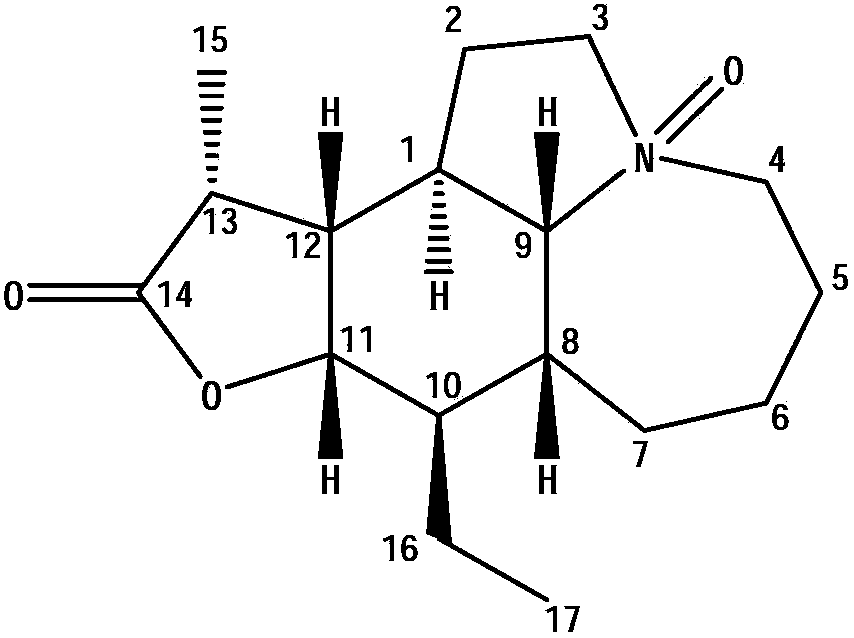
Stemona root alkaloid monomer components and applications thereof
A technology of alkaloids and monomers, applied in drug combinations, digestive system, muscular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1: Separation of Alkaloid Monomer Components
[0011] (1) The preparation of the total alkaloid extract of Herba Melonifolia
[0012] Take 5kg of herbal medicines, crush them, add 10L of 95% ethanol to reflux and extract twice, each time for two hours, combine the extracts, concentrate to dryness, add 3L of distilled water to dissolve, adjust the pH value to 1-2 with 4M concentrated hydrochloric acid, and statically Suction filtration after 12-16h. The filtrate was adjusted to pH 9-11 with concentrated ammonia water or aqueous sodium hydroxide solution, and then extracted twice with 5 L of chloroform, and the extracts were combined and concentrated to dryness to obtain the total alkaloid extract (75 g).
[0013] (2) Separation and identification of effective monomer components in the total alkaloid extracts
[0014] By macroporous resin column chromatography, ethanol-water gradient elution (20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100% %), collected once pe...
Embodiment 2
[0033] Embodiment 2: The improved Ellman method measures the anti-acetylcholinesterase activity of alkaloid monomer components
[0034] The improved Ellman method was used to determine the inhibitory rate of acetylcholinesterase compound 1-4 of the alkaloid monomer component of basilicum alkaloids, and huperzine A was used as the positive control drug. The specific experimental process is as follows:
[0035] Use a 96-well microtiter plate to determine the inhibitory activity of the sample on the enzyme. Add 140 μL of 0.1M phosphate buffer (pH=8.0) to each well of the sample group, and then add 20 μL of the sample. Add 160 μL of phosphate buffer to the blank well without adding For any sample, 20 μL of 0.1 mg / mL physostigmine and 140 μL of phosphate buffer were added to the positive control control group. Add 15 μL of acetylcholinesterase, mix well and incubate at 4°C for 20 min, then add 10 μL of 0.01 mM DTNB (5,5-dithiobis(2-nitrobenzoic acid)) and 10 μL of 0.075 mM ATCI ( ...
Embodiment 3
[0040] Embodiment 3: Morris water maze experiment
[0041] 110 healthy Kunming male mice were randomly divided into 11 groups: normal control group, model control group, compound 1-4 high-dose group (1mg / kg), compound 1-4 low-dose group (0.5mg / kg) and donepezil Control group (0.1 mg / kg). Except the normal control group and the model control group were intraperitoneally injected with the same amount of normal saline, the mice in the other groups were injected intraperitoneally once a day at a fixed time for 5 consecutive days. After 2 days of administration, water maze positioning navigation training was carried out. Except for the normal control group, which was intraperitoneally injected with the same amount of normal saline, the mice in the other groups were intraperitoneally injected with 2 mg / kg of scopolamine before training. Positioning and navigation training was carried out 1 hour after the last administration, and a space exploration test was carried out 24 hours la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com