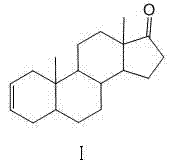
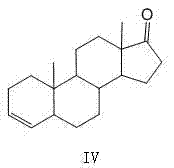
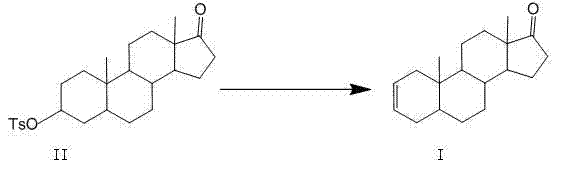
Industrial production method of 5 alpha-androst-2-ene-17-one
A technology for androstane and epiandrostone, which is applied in the field of 5α-androst-2-en-17-one, can solve the problems of complicated operation, corrosive pipeline, high cost and the like, and achieves simple and easy-to-obtain raw materials and pollution of three wastes. Less and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of epiandrosterone p-toluenesulfonate
[0052]
[0053] Dissolve 100g (0.344mol) of epiandrosterone in a mixed solvent of 100ml of pyridine and 200ml of dichloromethane, add 120g (0.63mol) of p-toluenesulfonyl chloride in portions under stirring and cooling with ice water, and react overnight at room temperature. Recover dichloromethane, add 1000ml of ice water to the residue, mash, filter, wash the filter cake with water until there is no pyridine smell, and obtain 148.5g of off-white solid epiandrosterone p-toluenesulfonate, yield 97%, mp163℃ ~165°C.
[0054] Refer to Zhou Mi, Lin Rong, et al. Study on the synthesis of pipecuronium bromide,[J]. Chinese Journal of Medicinal Chemistry, 2008, 18 (2): 115-118 introduced method preparation, the present invention is incorporated by reference.
[0055]
Embodiment 2
[0057] Preparation of 5α-androst-2-en-17-one
[0058] Add 2-picoline (140L, 1422.4mol) into the reaction kettle, add epiandrosterone p-toluenesulfonate (100kg, 225.2mol), stir and heat to about 128°C ~ reflux temperature until the reaction is complete, then decompress Recover 2-picoline by distillation, cool to room temperature, add 1% dilute sulfuric acid to the reaction solution to adjust the pH to 6-8, filter with suction, wash with water, drain and directly add 80% ethanol (100L) without drying, stir and reflux to completely dissolved. Slowly stirred and cooled to 20°C-35°C, centrifugally filtered to dryness, and vacuum-dried at 50°C to obtain 56.9 kg of off-white solid, yield 94.0%, mp 103°C-106°C, HPLC purity ≥99.0%.
[0059]
Embodiment 3
[0061] Preparation of 5α-androst-2-en-17-one
[0062] Add 3-picoline (140L, 1422.3mol) into the reaction kettle, add epiandrosterone p-toluenesulfonate (100kg, 225.2mol), stir and heat, reflux reaction, after the reaction is complete, 3-methylpyridine is recovered by vacuum distillation Pyridine, cool to 20℃~35℃, add 1% dilute sulfuric acid to the reaction solution to adjust the pH to 6~8, filter with suction, wash with water, drain and directly add 80% ethanol (100L) without drying, stir and heat up to reflux until completely dissolved . Slowly stirred and cooled to 20°C-35°C, centrifugally filtered to dryness, and vacuum-dried at 50°C to obtain 57.2 kg of off-white solid with a yield of 92.5%, mp 103-106°C, and HPLC purity ≥99.0%.
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com