Preparation method of gefitinib
A technology of gefitinib and compounds, which is applied in the field of innovative drug preparation, can solve the problems of chlorination pollution, long total route, etc., and achieve the effects of low environmental pollution, simple post-processing, safe, reliable and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
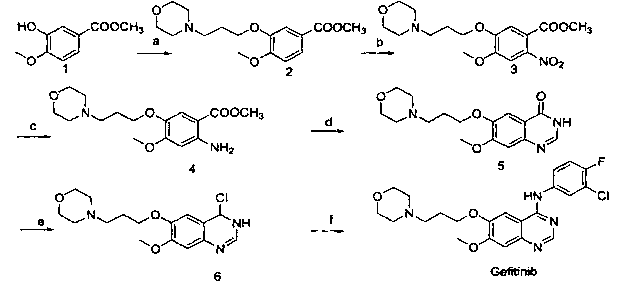
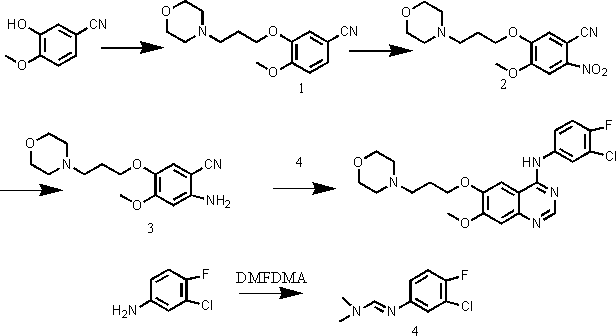
[0033] Embodiment 1, a kind of preparation method of gefitinib, this method comprises the steps:
[0034] (1) Using 3-hydroxy-4-methoxybenzocyanide and 4-(3-chloropropyl)morpholine as raw materials, react under the action of an appropriate solvent and base to prepare compound 1, namely 4-(3- Chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline;
[0035] (2) Compound 1 was nitrated to obtain compound 2, methyl 2-nitro-4-methoxy-5-[3-(4-morpholinyl)propoxy]benzoate;
[0036] (3) Compound 2 was reduced to obtain compound 3, namely methyl 2-amino-4-methoxy-5-[3-(4-morpholinyl)propoxy]benzoate;
[0037] (4) React 3-chloro-4-fluoroaniline with N,N-dimethylformamide dimethyl acetal to obtain compound 4, namely (3-chloro-4-fluorophenyl)-N,N-dimethyl dimethylimine;
[0038] (5) Ring closure of compound 3 and compound 4 in a solvent to obtain gefitinib.
Embodiment 2
[0039] Example 2, the preparation method of gefitinib described in Example 1, in step (1): the reaction solvent is selected from dimethyl sulfoxide, dimethylformamide, dimethylacetamide, toluene and dimethicone One or more mixtures of toluene; the base is an inorganic base or an organic base, wherein: the inorganic base is selected from sodium carbonate, sodium bicarbonate, potassium carbonate, and potassium bicarbonate; The base is one or a mixture of trimethylamine, triethylamine, tripropylamine and tri-n-butylamine.
Embodiment 3
[0040] Embodiment 3, the preparation method of gefitinib described in embodiment 1 or 2, in step (2): the mass fraction of nitric acid is 50%-85%; The reaction time is 1-24 hours, and the reaction temperature is 0 -80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com