Capecitabine pharmaceutical composition and preparation method thereof
A technology of capecitabine and its composition, applied in the field of pharmaceutical preparations, to achieve the effect of being beneficial to the treatment of gastric cancer, suitable for large-scale production, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
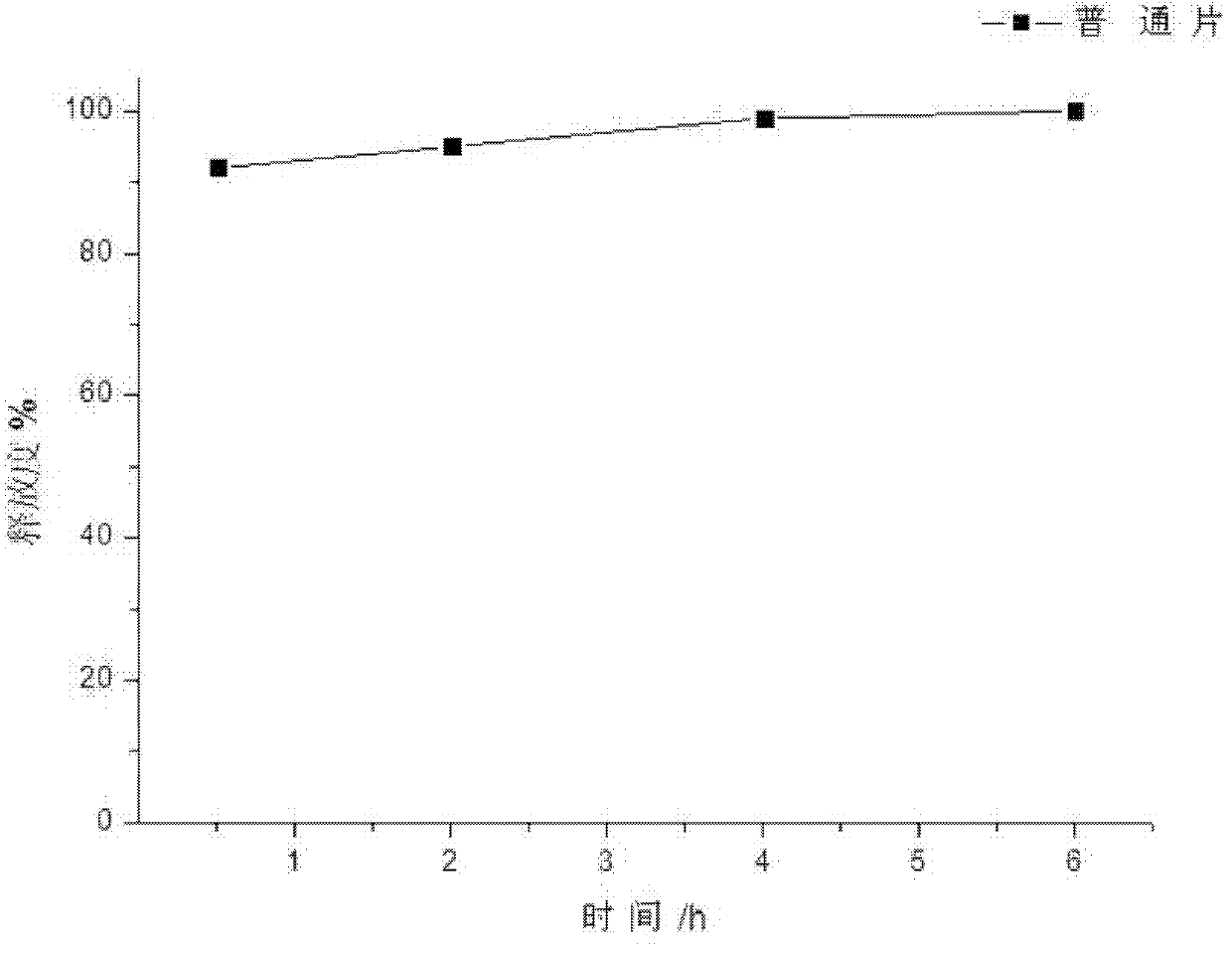
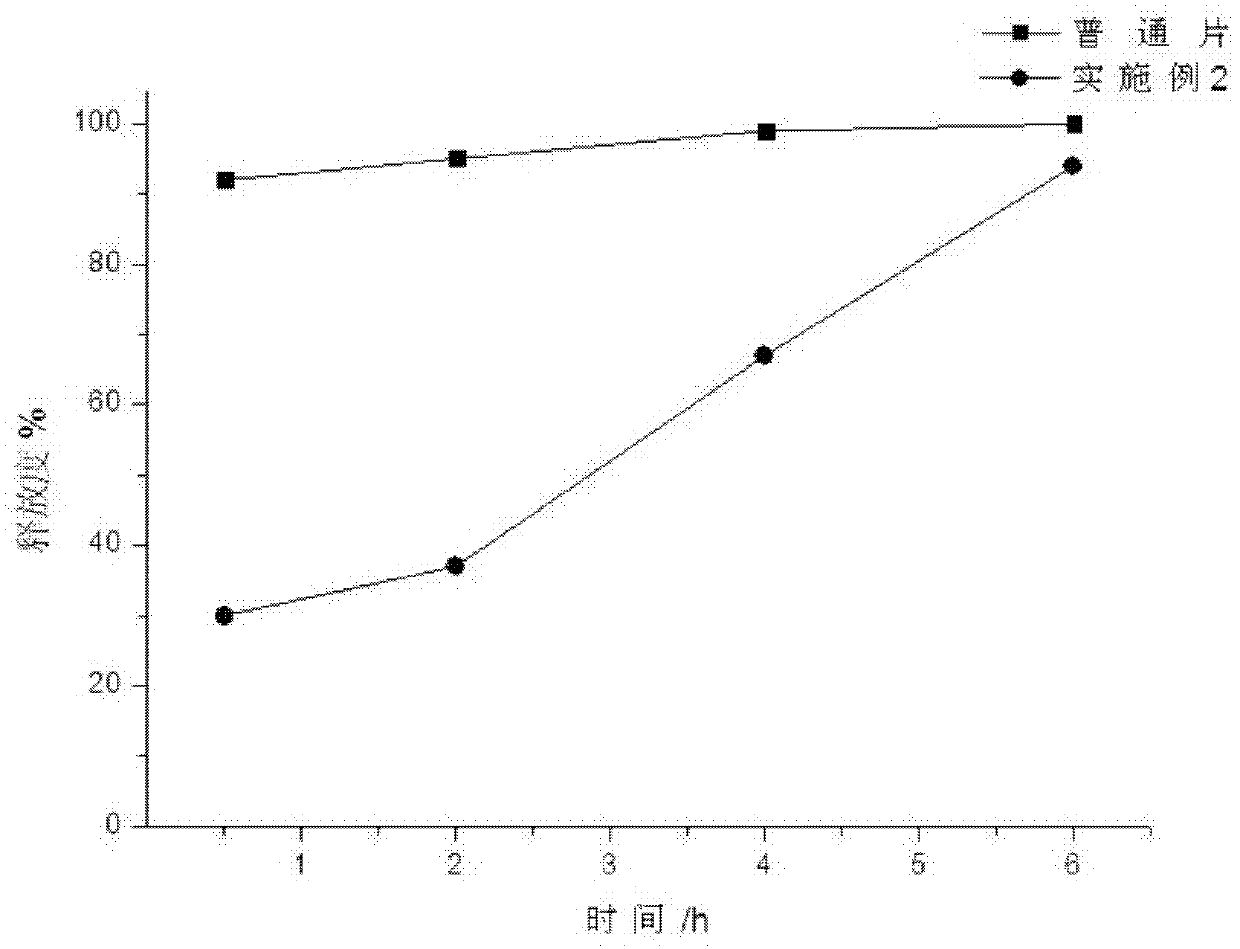
Image
Examples
Embodiment 1
[0033] A capecitabine intragastric floating tablet, the main components of which are:
[0034] name
[0035] Preparation process: Pass capecitabine, hydroxypropyl methylcellulose, calcium carbonate, stearyl alcohol, and polyethylene glycol through 80 mesh sieves, mix evenly according to the above ratio, add an appropriate amount of purified water, and use pharmaceutical industry The known method is used for granulation, and the prepared granules are dried in a drying oven at 60° C. until the water content is 1%, and the granules are sized with a 30-mesh sieve. After mixing the prepared granules and magnesium stearate, press into tablets, the tablet hardness is controlled at 5kg / cm 2 , that is, this product.
Embodiment 2
[0037] A capecitabine intragastric floating tablet, the main components of which are:
[0038] name
[0039] Preparation process: Pass capecitabine, carbomer, magnesium carbonate, cetyl alcohol, and lactose through 80-mesh sieve respectively, mix evenly according to the above-mentioned ratio, add an appropriate amount of purified water, and granulate using a known method in the pharmaceutical industry. The prepared granules were dried in a drying oven at 60°C until the moisture content was 0.5%, and sieved with a 30-mesh sieve for sizing. After mixing the prepared granules and zinc stearate, press into tablets, the tablet hardness is controlled at 6kg / cm 2 , that is, this product.
Embodiment 3
[0041] A capecitabine intragastric floating tablet, the main components of which are:
[0042] name
[0043] Preparation process: Pass capecitabine, hydroxypropyl methylcellulose, sodium bicarbonate, stearyl alcohol, and polyvinylpyrrolidone through 80-mesh sieves, mix evenly according to the above ratio, add an appropriate amount of purified water, and use pharmaceutical industry The known method is used for granulation, and the prepared granules are dried in a drying oven at 60° C. until the water content is 0.3%, and the granules are sized with a 30-mesh sieve. After mixing the prepared granules and stearic acid, press into tablets, the tablet hardness is controlled at 4kg / cm 2 , that is, this product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com