Expanded graphite composite heat storage material and its preparation method and application
A technology of expanded graphite and composite materials, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of dehydration kinetics speed restricting large-scale application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
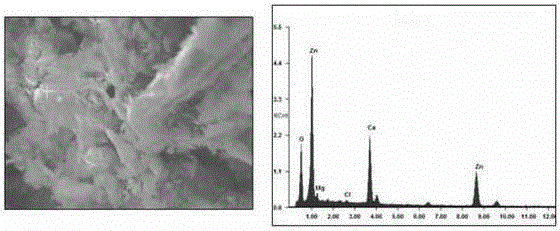
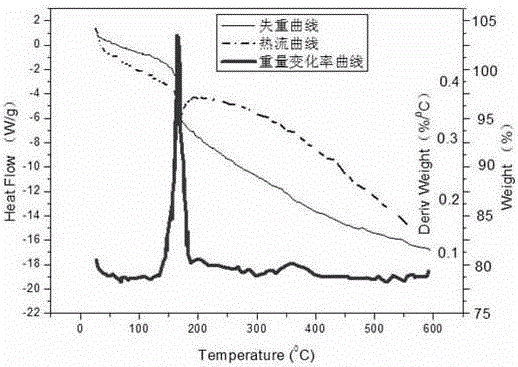
[0022] Add 1000mL of distilled water to the beaker and heat to 293K, then add enough ammonium chloride into the beaker, stir well, filter to obtain a saturated ammonium chloride solution, weigh 144mg of calcium hydroxide and add it to the beaker, stir to make it fully Dissolve; weigh 162mg of zinc oxide and add it to the calcium hydroxide solution, stir continuously, and react continuously for 12 hours to obtain a milky white suspension; add 200mg of powdered expanded graphite to the beaker, and ultrasonically vibrate for 24 hours at a temperature of 293K; then After the solution was left to stand for about 12 hours at a temperature of 293K, the beaker was placed at a temperature of 353K for about 10 hours to discharge ammonia and water, and after drying, the expanded graphite was taken out and washed with distilled water, heated to 353K, dried for 10 hours, and washed with circulating water and drying repeated 2-3 times to obtain the final expanded graphite-based calcium-based...
Embodiment 2
[0024] Add 1000mL of distilled water to the beaker and heat to 293K, then add enough ammonium chloride to the beaker, stir well, filter to obtain a saturated ammonium chloride solution, weigh 74mg of calcium hydroxide and add it to the beaker, and stir to make It is fully dissolved; weigh 162mg of zinc oxide and add it to the prepared calcium hydroxide solution, stir continuously, and react continuously for 12 hours to obtain a milky white suspension; place 200mg of powdered expanded graphite in a beaker and vibrate ultrasonically at a temperature of 293K 24h; then put the solution at a temperature of 293K for about 12h, place the beaker at a temperature of 353K for about 10h to discharge ammonia and water, take out the expanded graphite after drying and clean the surface; the taken out expanded graphite Cool to room temperature and wash with distilled water; heat the sample to a temperature of 353K for about 10h to dry the sample. The final expanded graphite-based Ca x Zn y...
Embodiment 3
[0026] Add 1000mL of distilled water to the beaker and heat to 293K, then add enough ammonium chloride to the beaker, stir well, filter to obtain a saturated ammonium chloride solution, weigh 74mg of calcium hydroxide and add it to the beaker, and stir to make It is fully dissolved; weigh 150 mg of nickel oxide and add it to the prepared calcium hydroxide solution, stir continuously, and react continuously for 12 hours to obtain a milky white suspension; place powdered expanded graphite in a beaker, and ultrasonically vibrate at a temperature of 293K for 24 hours Then the solution was left to stand at a temperature of 293K for about 12h, and the beaker was placed at a temperature of 353K for about 10h to exhaust ammonia and water, and after drying, the expanded graphite was taken out and the surface was cleaned; the expanded graphite taken out was cooled to room temperature, and washed with distilled water; the sample was heated to a temperature of 353K for about 10h, and the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com