Vinyl sulfone-substituted cysteine-N-carboxyanhydride, polymer thereof and application of polymer
A technology of cysteine hydrochloride and cysteine, applied in the field of polymer materials, can solve problems such as weak physical and mechanical properties, complicated preparation process, and strict conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
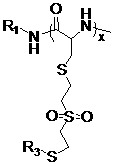
[0073] Example 1 Synthesis of vinylsulfone substituted cysteine N-carboxyl internal anhydride (VSCys-NCA).
[0074] (1) Dissolve 4.45 g (25 mmol) of cysteine hydrochloride in 80 ml of methanol, drop by drop into the methanol solution of divinyl sulfone (12.55 ml, 125 mmol); stir in an oil bath at 50°C and react for 100 hours The resulting solution was concentrated under reduced pressure and precipitated with ethyl acetate, filtered, and dried to obtain 6.58 g of white solid powder (vinylsulfone substituted L-cysteine, VSCys), with a yield of 94.4%.
[0075] (2) Weigh VSCys (3.75 g, 13.6 mmol) in a dry three-necked round bottom flask, add anhydrous THF (100 ml), then add pinene (α-pinene) (5.0 ml, 31.5 mmol), The reaction system was placed in an oil bath at 50°C for 30 minutes, and then solid triphosgene (2.02 g, 6.8 mmol) was added; the reaction temperature was increased to 70°C, and after 1 hour of reaction, the crude product of VSCys-NCA was obtained by precipitation with pet...
Embodiment 2
[0077] Example 2: Synthesis of vinylsulfone substituted cysteine N-carboxyl internal anhydride (VSCys-NCA)
[0078] (1) Dissolve 4.45 g (25 mmol) of cysteine hydrochloride in 80 ml of methanol, drop by drop into the methanol solution of divinyl sulfone (50.2 ml, 500 mmol); stir and react in an oil bath at 40°C for 60 hours The resulting solution was concentrated under reduced pressure and precipitated with ethyl acetate, filtered, and dried to obtain 6.97 g of white solid powder (vinylsulfone substituted L-cysteine, VSCys) with a yield of 95.7%.
[0079] (2) Weigh VSCys (3.75 g, 13.6 mmol) in a dry three-necked round bottom flask, add anhydrous THF (100 ml), then add pinene (α-pinene) (6.5 ml, 40.8 mmol), The reaction system was placed in an oil bath at 50°C for 30 minutes, and then solid triphosgene (2.02 g, 13.6 mmol) was added; the reaction temperature was increased to 70°C, and after 1 hour of reaction, the crude product of VSCys-NCA was obtained by precipitation with petro...
Embodiment 3
[0080] Example 3 Preparation of polyamino acid random copolymer by copolymerization of VSCys-NCA and other α-amino acid-N-carboxyl internal acid anhydride
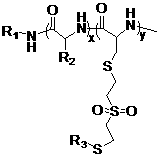
[0081] According to the ratio of each component in Table 1, dissolve other α-amino acid-N-carboxyl internal acid anhydride and VSCys-NCA solid in anhydrous DMF in a glove box; add the small molecule initiator propylene trimethylsilazane The reaction of alkane in DMF solution was placed in an oil bath at 40°C for 48 h under anhydrous conditions, and the reaction was terminated with two drops of acetic acid; the polymer solution was precipitated in glacial ether, filtered and vacuum dried at room temperature to obtain the product polyamino acid.
[0082] Attached figure 2 Schematic diagram of the above random copolymerization reaction. The reaction was monitored by infrared: After the reaction was carried out for 48 h, the carbonyl absorption peak of the two monomer anhydrides in the infrared spectrum was 1869 cm -1 (VS-Cys NCA)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com