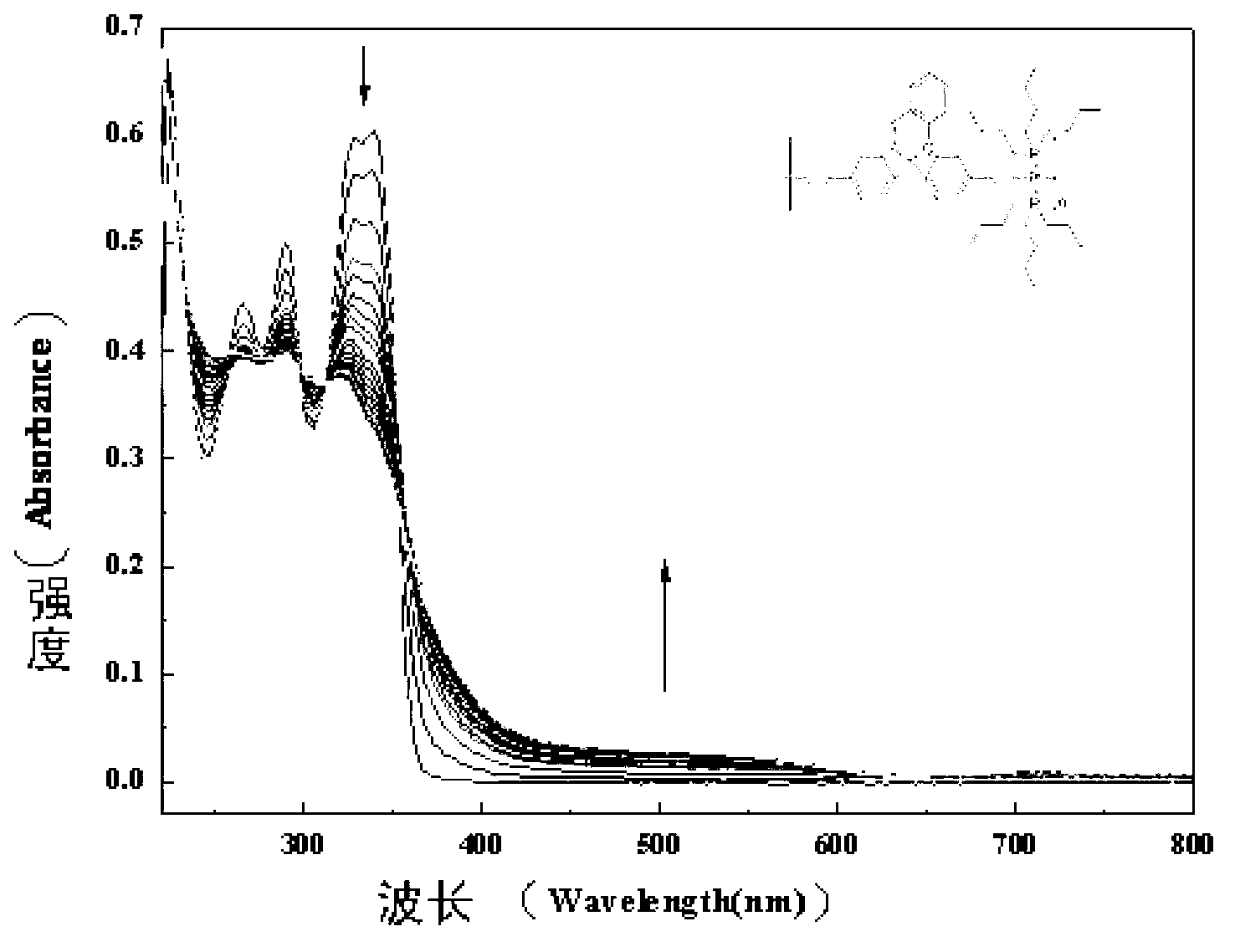
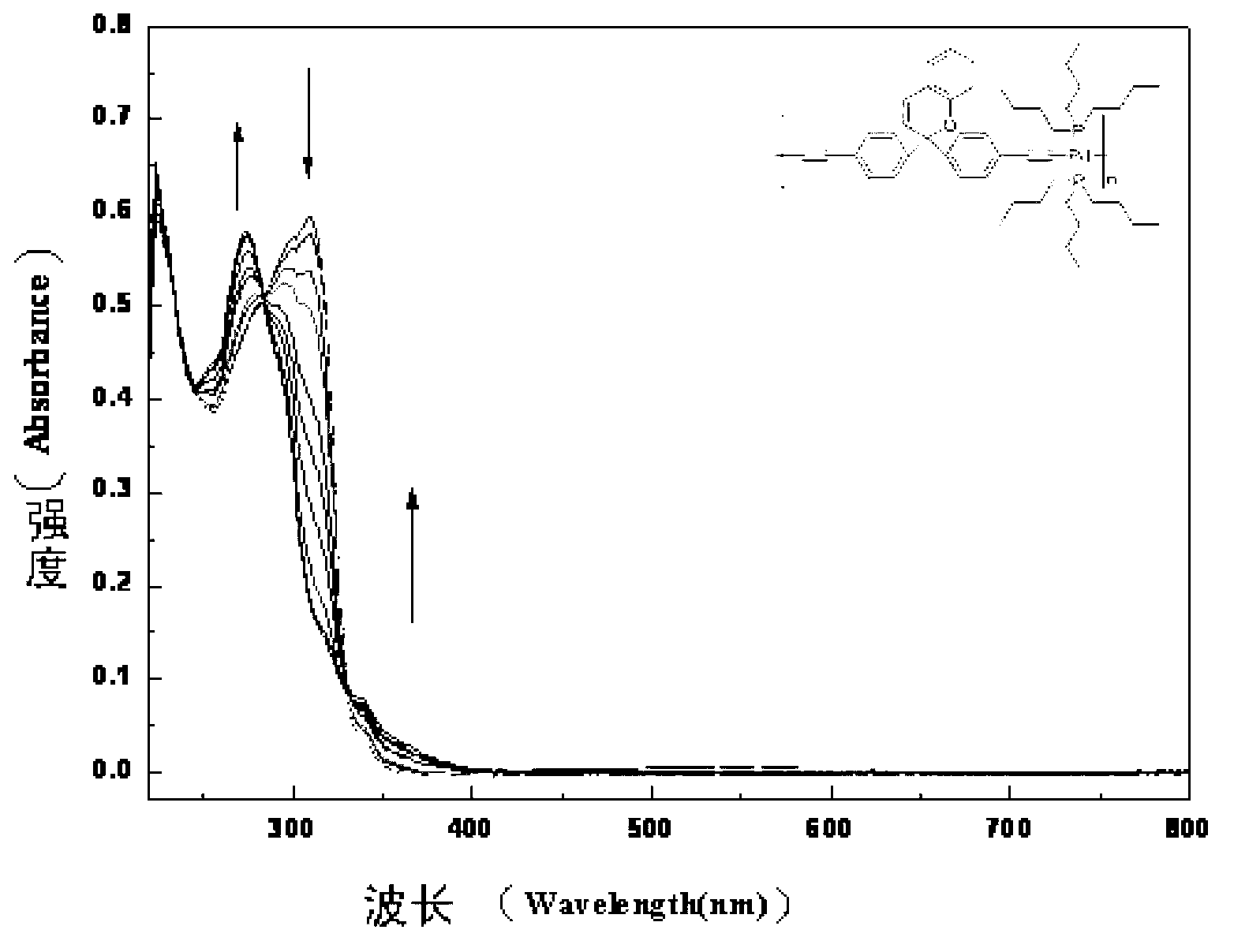
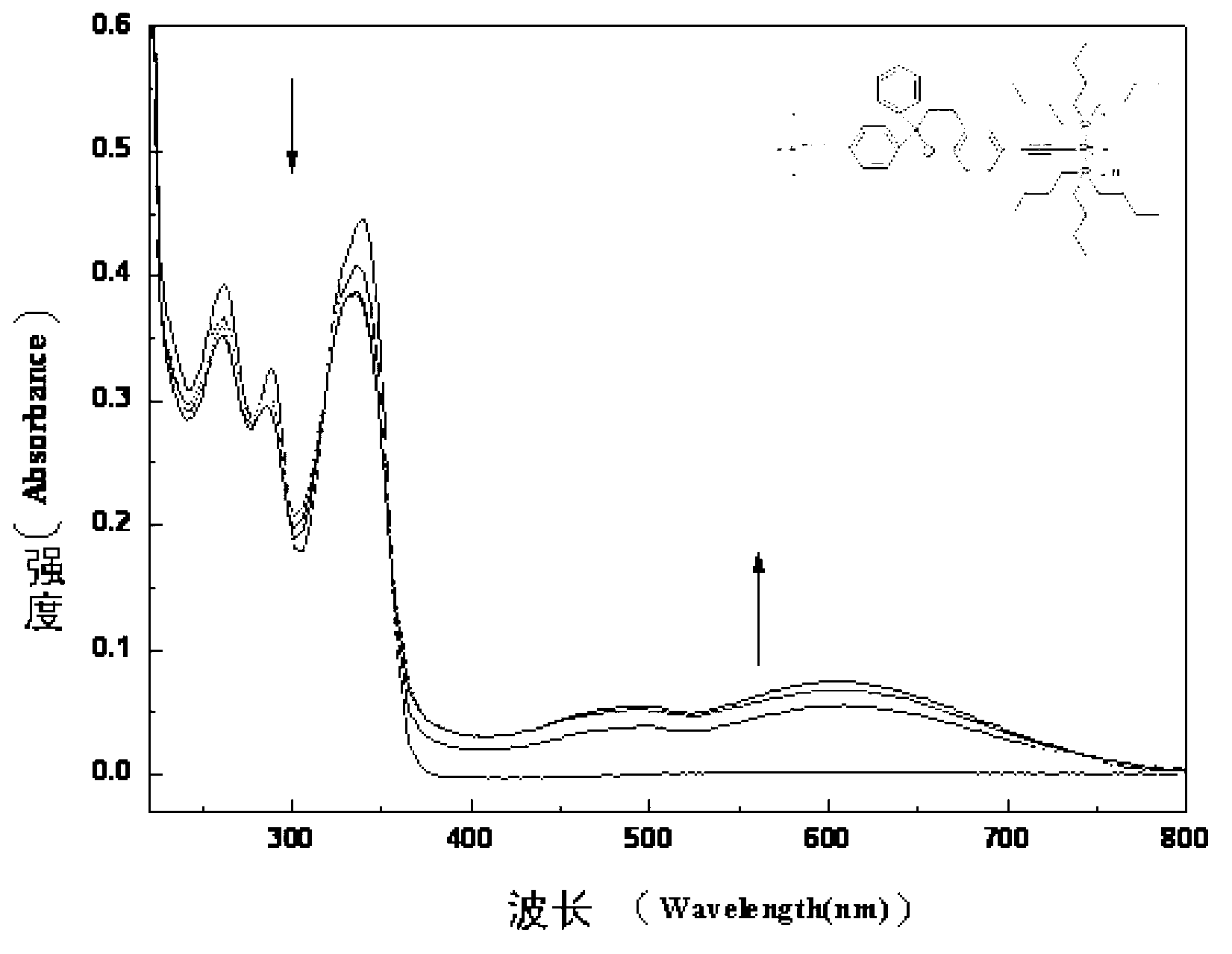
Photochromic polymers containing spiro group and its synthetic method
A photochromic and polymer technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of low fatigue resistance and low application range, and achieve good photochromic performance, high yield, and simple synthesis process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthetic method of photochromic polymer 1 containing spiro group, comprises the following steps:
[0046] Step 1. Using trimethylsilylacetylene as a raw material, react with butyl lithium and 4,4'-dibromobenzophenone at -78°C under nitrogen protection conditions to generate hydroxyl-containing terminal alkyne bonds in equal amounts compound;
[0047] The reaction formula is:
[0048]
[0049] The specific operation is: add 1.67g (17mmol) TMSA to a 250ml three-necked flask, add 30ml anhydrous tetrahydrofuran, cool to -78°C, slowly add 6.5ml (15.6mmol) of 2.4M butyllithium solution dropwise, and keep warm for 30min. 4.82 g (14.2 mmol) of 4,4'-dibromobenzophenone was added. After 12 hours, deionized water and 3.7 g (14.2 mmol) of tetrabutylammonium fluoride were added. The organic layer was extracted with dichloromethane, spin-dried and passed through the column with dichloromethane:petroleum ether=3:1 to obtain a light yellow solid m=5.23g, η=98%;
[0050] The...
Embodiment 2
[0087] The synthetic method of photochromic polymer 2 containing spiro group, comprises the following steps:
[0088] Step 1. Using trimethylsilylacetylene as a raw material, under the condition of nitrogen protection at -78°C, through the reaction of butyl lithium and 4-bromobenzophenone in equal amounts to generate a hydroxyl-containing terminal alkyne bond compound;
[0089] The reaction formula is:
[0090]
[0091] The specific operation is: add 1.67g (17mmol) TMSA to a 250ml three-necked flask, add 2.4g (24mmol) TMSA to a 250ml three-necked flask, add 30ml anhydrous tetrahydrofuran, and cool to -78°C. Add 8.8ml (22mmol) of 2.4M butyllithium solution dropwise slowly, and keep warm for 30min. Add 5.22 g (20 mmol) of 4 dibromobenzophenone. After 12 hours, deionized water and 5.23 g (20 mmol) of tetrabutylammonium fluoride were added. The organic layer was extracted with dichloromethane, spin-dried and passed through the column with dichloromethane:petroleum ether=3:1 ...
Embodiment 3
[0128] The synthetic method of photochromic polymer 3 containing spiro group, comprises the following steps:
[0129] Step 1, the same as Step 1 in Example 2 to obtain a hydroxyl-containing terminal acetylenic bond compound.
[0130] Step 2, the product obtained in step 1 is dehydrated with 4,4'-biphenyldiphenol under the catalysis of pyridine p-toluenesulfonate with a mole fraction ratio of 5% through three times the equivalent of dehydrating agent trimethyl ortho acid ring-closing reaction to obtain a corresponding spiro-type photochromic unit;
[0131] The reaction formula is:
[0132]
[0133] The specific operation is: add 1.5g (5.2mmol) of bromide, 0.46g (2.5mmol) of 4,4'-biphenol, 0.07g (0.28mmol) of pyridine p-toluenesulfonate (PPTS) into the reaction tube, nitrogen Under atmosphere, 1.7 ml (15.6 mmol) of trimethyl orthoacid was added, and then 25 ml of 1,2-dichloroethane was added, and the mixture was stirred at 85° C. for 12 hours. Pass through the column with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com