Esomeprazole magnesium preparation method
A technology of esomeprazole and magnesium alkoxide is applied in the field of drug synthesis, and can solve the problems such as difficulty in obtaining raw materials, unsuitability for industrialized production, and many steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
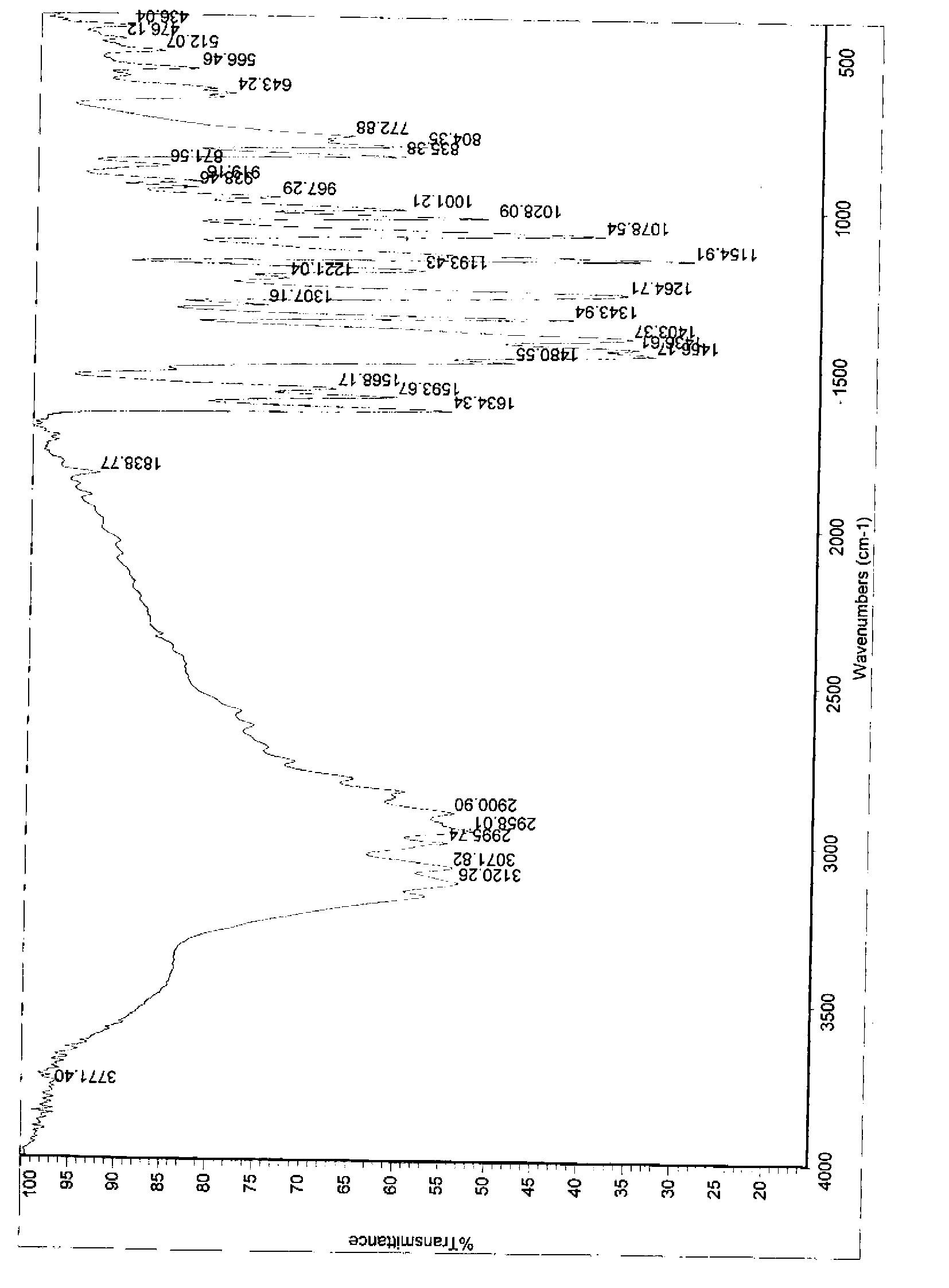
[0090] Add 1kg of 2-mercapto-5-methoxybenzimidazole (5.56mol) to dissolve in 12L of methanol, add dropwise 478g of sodium hydroxide (12mol) aqueous solution, drop to clear liquid, stop adding sodium hydroxide, and add 1.08 kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (5.84mol), and then continue to add the remaining sodium hydroxide dropwise, after the drop is complete, heat up to 85°C, stir and reflux 5h. After the reaction is over, recover methanol under reduced pressure, adjust the acidity of the remaining aqueous solution to pH = 8 with acetic acid, extract the aqueous solution three times with 3L dichloromethane, recover dichloromethane under reduced pressure, add 1.1L ethyl acetate to the residue and heat to reflux , cooled, stirred and crystallized at room temperature. Suction filtration and washing with ethyl acetate gave 1.76 kg of omeprazole sulfide white solid with a yield of 96%, a purity of 99.8%, and m.p.121-122°C. IR(KBr), v / cm -1 : 3120,...
Embodiment 2
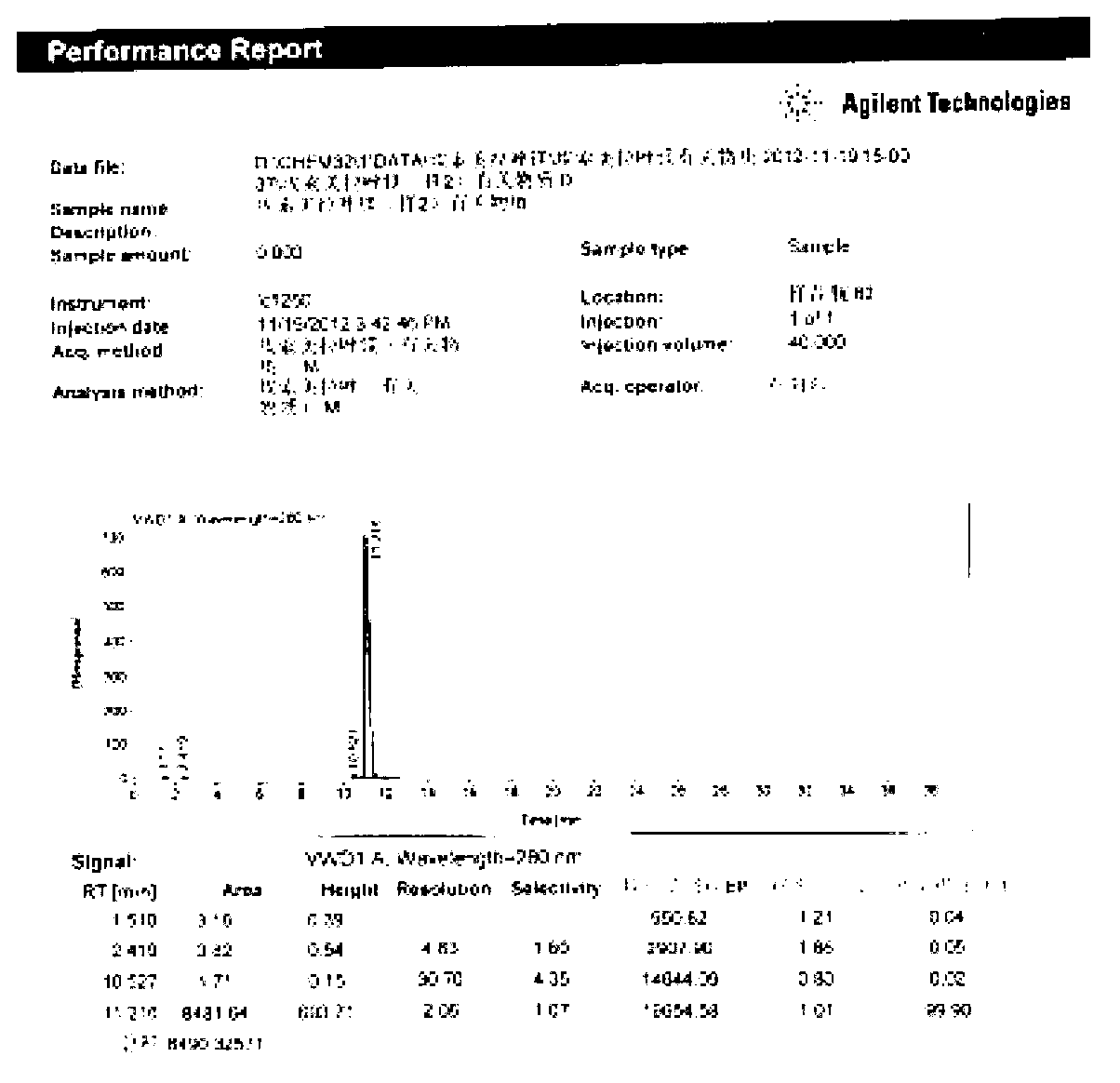
[0095] Add 1kg of 2-mercapto-5-methoxybenzimidazole (5.56mol) to dissolve in 12L of methanol, add dropwise 478g of sodium hydroxide (12mol) aqueous solution, drop to clear liquid, stop adding sodium hydroxide, and add 1.08 kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (5.84mol), and then continue to add the remaining sodium hydroxide dropwise, after the drop is complete, heat up to 85°C, stir and reflux 5h. After the reaction is over, recover methanol under reduced pressure, adjust the acidity of the remaining aqueous solution to pH = 8 with acetic acid, extract the aqueous solution three times with 3 L of dichloromethane, recover the dichloromethane under reduced pressure, add 1.1 L of ethyl acetate to the resulting residue and heat Reflux, cool, stir and crystallize at room temperature. After suction filtration and washing with ethyl acetate, 1.74 kg of omeprazole sulfide was obtained as a white solid with a yield of 95%, a purity of 99.5%, and m.p.121-1...
Embodiment 3
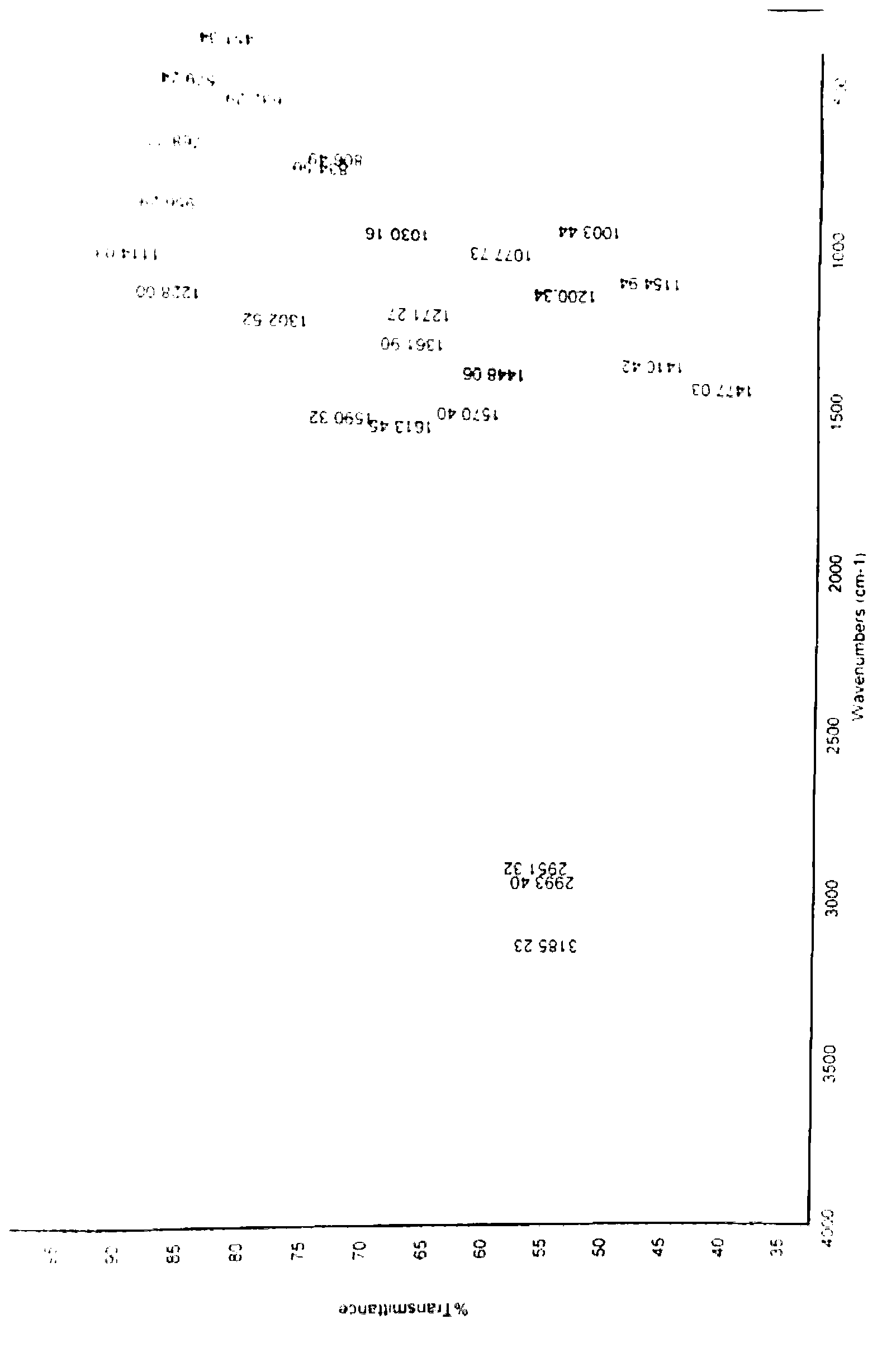
[0099] Add 1kg of 2-mercapto-5-methoxybenzimidazole (5.56mol) to dissolve in 12L of methanol, add dropwise 478g of sodium hydroxide (12mol) aqueous solution, drop to clear liquid, stop adding sodium hydroxide, and add 1.08 kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (5.84mol), and then continue to add the remaining sodium hydroxide dropwise, after the drop is complete, heat up to 85°C, stir and reflux 5h. After the reaction is over, recover methanol under reduced pressure, adjust the acidity of the remaining aqueous solution to pH = 8 with acetic acid, extract the aqueous solution three times with 3L dichloromethane, recover dichloromethane under reduced pressure, add 1.1L ethyl acetate to the residue and heat to reflux , cooled, stirred and crystallized at room temperature. After suction filtration and washing with ethyl acetate, 1.78 kg of omeprazole sulfide was obtained as a white solid with a yield of 97%, a purity of 99.5%, and m.p.121-122°C.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com