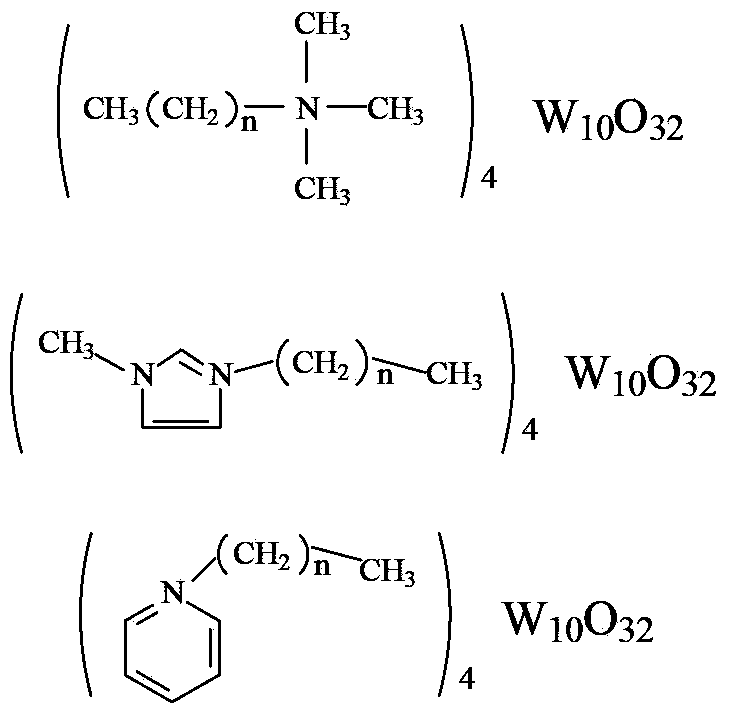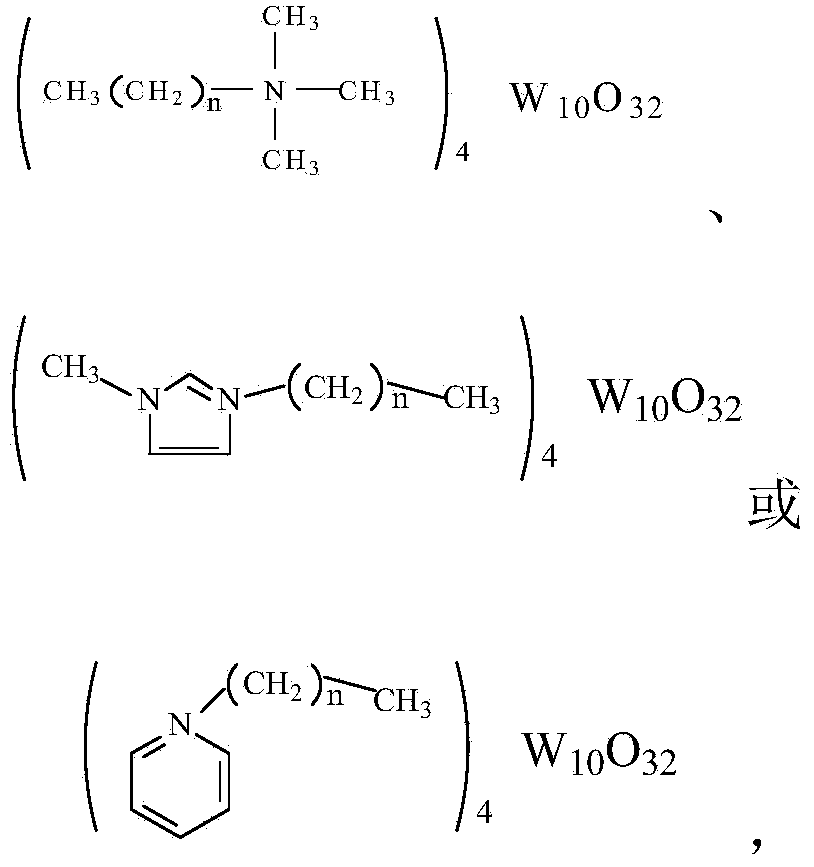Method for preparing 1,2-cyclohexanediol through carrying out catalytic oxidation on cyclohexene by using phase transfer catalyst
A technology of catalytic oxidation and cyclohexanediol, applied in hydroxyl addition preparation, organic chemistry, etc., can solve the problems of difficult separation and recovery, high production cost, and large consumption of ionic liquid, achieve good application prospects, and reduce combustion and explosion The effect of the danger of separation and the convenience of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of quaternary ammonium octyl trimethyl decapolytungstate:
[0021] Weigh 32.95g of sodium tungstate and add it into a beaker filled with 100mL of water to dissolve, stir magnetically at 60°C, adjust its pH to about 2 with 3mol / L hydrochloric acid, then slowly add 10.02g of Octyltrimethylammonium bromide in 10mL ethanol solution, the solution immediately appears white turbid. After the dropwise addition, the reaction was continued at 90°C for 0.5h. After the reaction, the white or light yellow solid was obtained by filtration, washed with water and ethanol, and dried in vacuum. The obtained product was detected by an organic elemental analyzer, and it was confirmed that it was the target product. Theoretical value: C=17.38%, H=3.46%, N=1.84%; actual value: C=17.29%, H=3.57%, N=1.81%.
Embodiment 2
[0023] Preparation of butyl trimethyl decapolytungstate quaternary ammonium salt:
[0024] Weigh 33.01g of sodium tungstate and add it into a beaker filled with 100mL of water to dissolve, stir magnetically at 60°C, adjust the pH value to about 2 with 3mol / L hydrochloric acid, then slowly add 7.85g 10mL ethanol solution of butyltrimethylammonium bromide, the solution immediately appeared white turbid. After the dropwise addition, the reaction was continued at 90°C for 0.5h. After the reaction, the white or light yellow solid was obtained by filtration, washed with water and ethanol, and dried in vacuum. The obtained product was detected by an organic elemental analyzer, and it was confirmed that it was the target product. Theoretical value: C=11.94%, H=2.58%, N=1.99%; actual value: C=11.89%, H=2.52%, N=2.03%.
Embodiment 3
[0026] Preparation of hexadecyl trimethyl decapolytungstate quaternary ammonium salt:
[0027] Weigh 32.98g of sodium tungstate and add it into a beaker filled with 100mL of water to dissolve, stir magnetically at 60°C, adjust the pH value to about 2 with 3mol / L hydrochloric acid, then slowly add 14.58g 10mL ethanol solution of cetyltrimethylammonium bromide, the solution immediately appears white turbid. After the dropwise addition, the reaction was continued at 90°C for 0.5h. After the reaction, the white or light yellow solid was obtained by filtration, washed with water and ethanol, and dried in vacuum. The obtained product was detected by an organic elemental analyzer, and it was confirmed that it was the target product. Theoretical value: C=26.16%, H=4.86%, N=1.61%; actual value: C=26.20%, H=4.79%, N=1.58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com