Photosensitive composition, cured film formed from same, and element having cured film
A technology of photosensitive composition and cured film, which is applied in the field of photosensitive composition, cured film formed therefrom, and components with cured film, which can solve the problems of reduced electrode conductivity, insufficient heat resistance or chemical resistance , Cured film coloring, transparency reduction and other problems, to achieve the effect of excellent moisture resistance and high heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
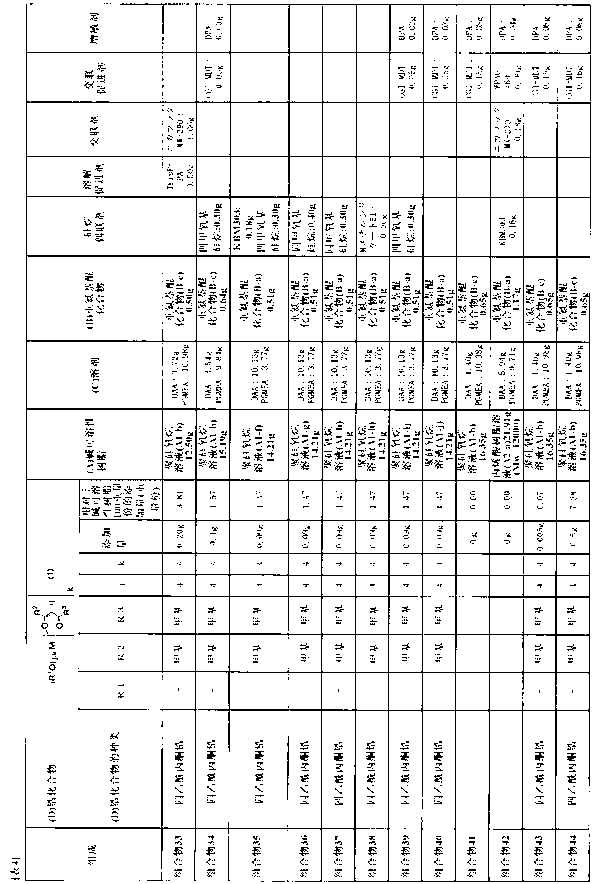
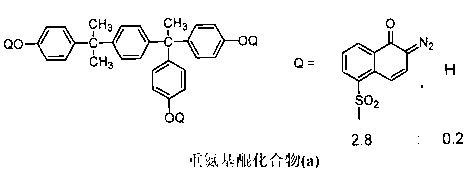
[0171] Examples are given below to describe the present invention more specifically, but the present invention is not limited by these Examples. In addition, among the compounds used, the abbreviated compounds are as follows.
[0172] DAA: diacetone alcohol
[0173] PGMEA: Propylene Glycol Monomethyl Ether Acetate
[0174] GBL: gamma-butyrolactone
[0175] EDM: diethylene glycol methyl ethyl ether
[0176] DPM: dipropylene glycol monomethyl ether.
[0177] In addition, the solid content concentration of the polysiloxane solution and the acrylic resin solution, and the weight average molecular weight (Mw) of the polysiloxane and the acrylic resin were obtained as follows.
[0178] (1) Solid content concentration
[0179] 1 g of the polysiloxane solution or the acrylic resin solution was weighed in an aluminum cup, and heated at 250° C. for 30 minutes using a hot plate to evaporate the liquid component. The solid content remaining in the heated aluminum cup was weighed to ...
Synthetic example 1
[0190] Synthesis example 1: Synthesis of polysiloxane solution (A1-a)
[0191] Add 81.72 g (0.60 mol) of methyltrimethoxysilane, 59.49 g (0.30 mol) of phenyltrimethoxysilane, (2-(3,4-epoxycyclohexyl) ethyl trimethyl Oxysilane 24.64g (0.10 mol), DAA 163.1g, while stirring at room temperature, was added with 10 minutes in water 55.8g phosphoric acid 0.54g (0.3% by weight relative to the added monomer) of phosphoric acid aqueous solution. Then After immersing the flask in a 40°C oil bath and stirring for 30 minutes, the oil bath was heated to 115°C in 30 minutes. One hour after the start of the temperature rise, the internal temperature of the solution reached 100°C, and it was heated and stirred for 1.5 hours (within Temperature is 100~110 ℃), obtain polysiloxane solution (A1-a). It should be noted that, during heating and stirring, flow nitrogen at 0.05L (liter) / min. Methanol, water as by-products in the reaction A total of 131 g was distilled off.
[0192] The solid content ...
Synthetic example 2
[0193] Synthesis Example 2: Synthesis of polysiloxane solution (A1-b)
[0194] Add 54.48 g (0.40 moles) of methyltrimethoxysilane, 99.15 g (0.50 moles) of phenyltrimethoxysilane, (2-(3,4-epoxycyclohexyl) ethyl trimethyl Oxysilane 24.64g (0.10 mol), DAA 179.5g, while stirring at room temperature, was added with 10 minutes in water 55.8g phosphoric acid 0.54g (0.3% by weight relative to the added monomer) of phosphoric acid aqueous solution. Then After immersing the flask in a 40°C oil bath and stirring for 30 minutes, the oil bath was heated to 115°C in 30 minutes. One hour after the start of the temperature rise, the internal temperature of the solution reached 100°C, and thus heated and stirred for 2 hours (within Temperature is 100~110 ℃) to obtain polysiloxane solution (A1-b). It should be noted that, during heating and stirring, nitrogen gas was circulated at 0.05L (liter) / min. Methanol and water, which were by-products in the reaction, were A total of 121 g was distilled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com