Method for preparing anidulafungin
A technology of anidulungin and protecting group, applied in the field of preparation of echinocandin antifungal drugs, achieving high yield and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
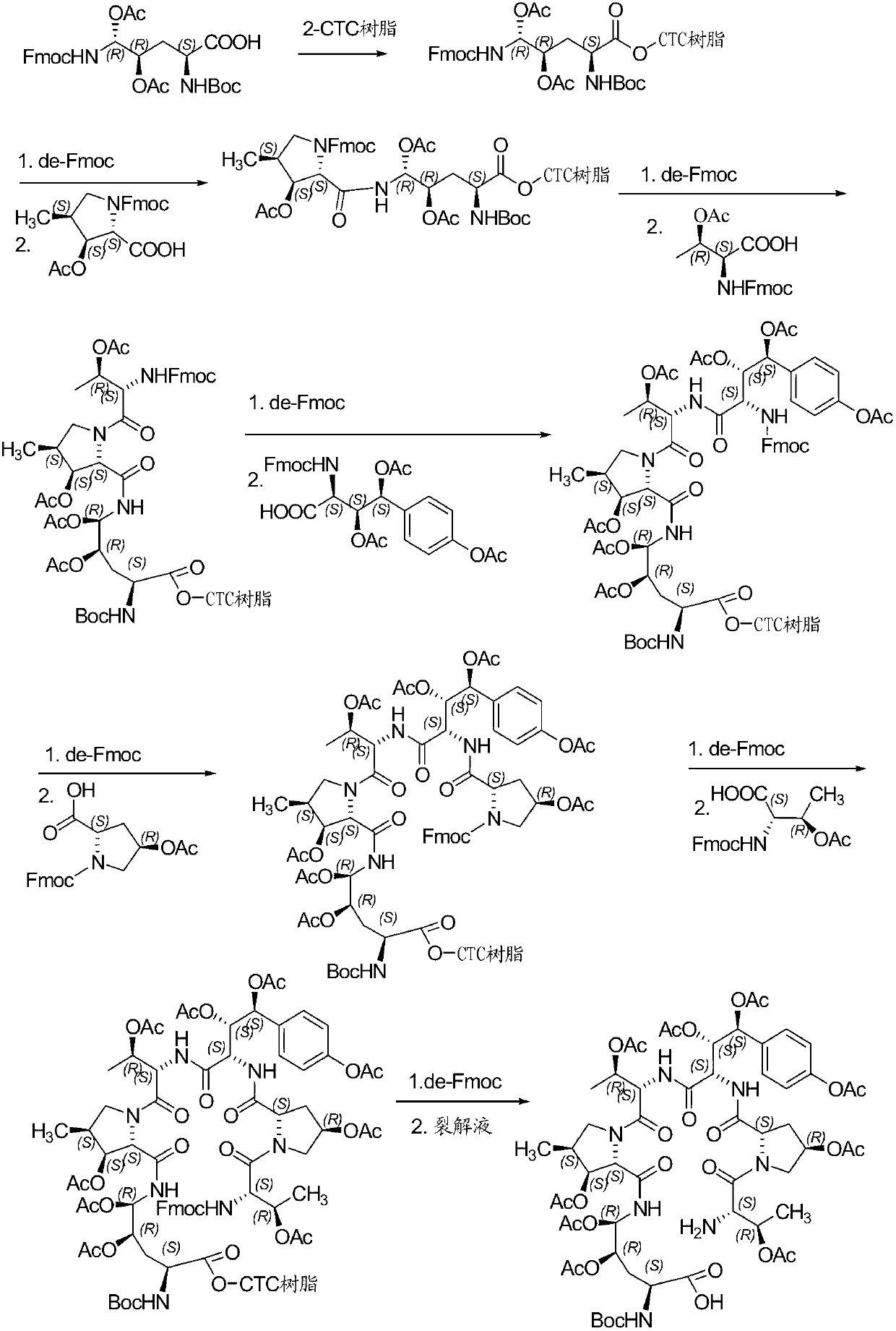
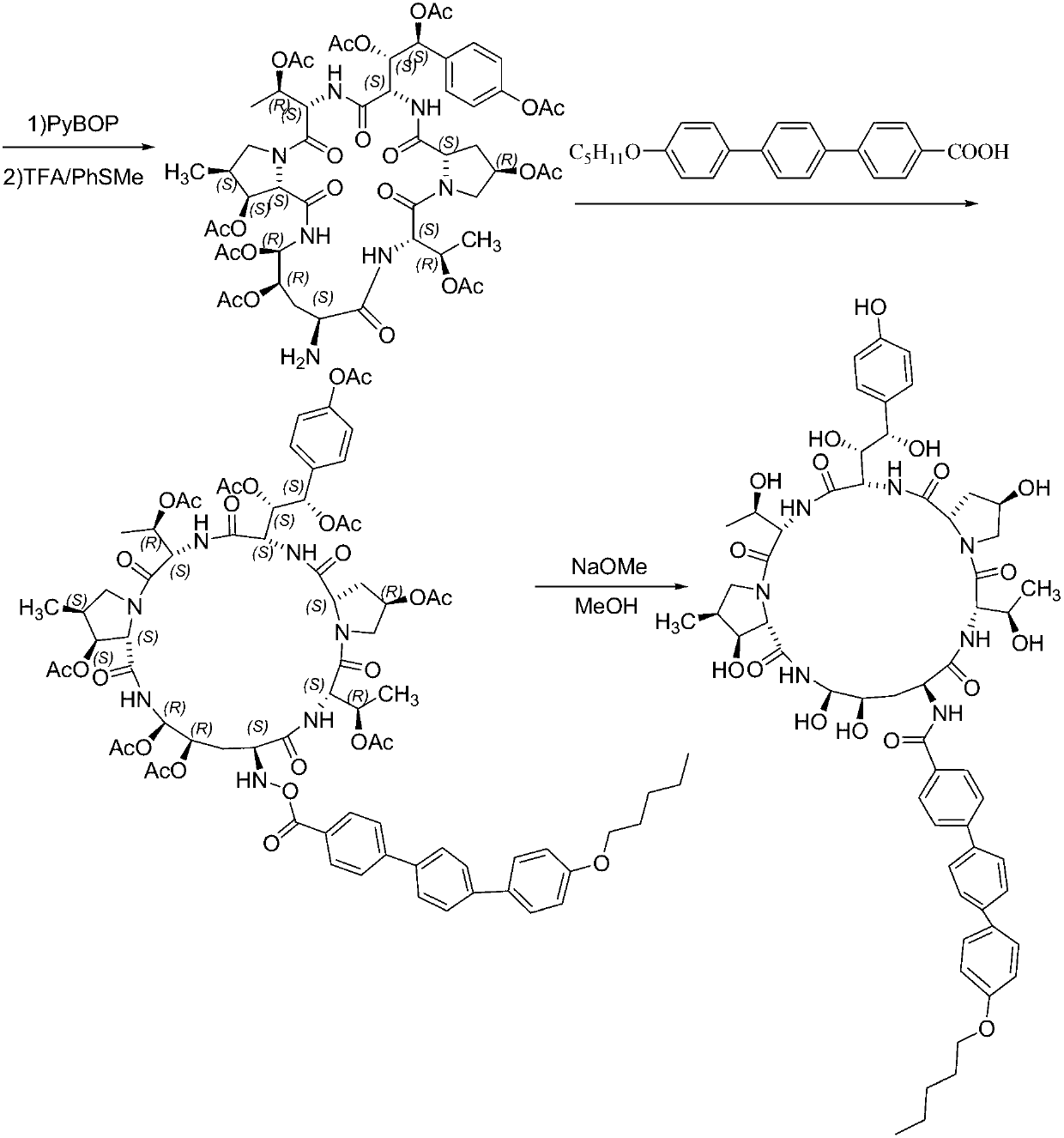
Embodiment 1
[0042] Embodiment 1: Synthesis of Fmoc-P-OH unnatural amino acid:
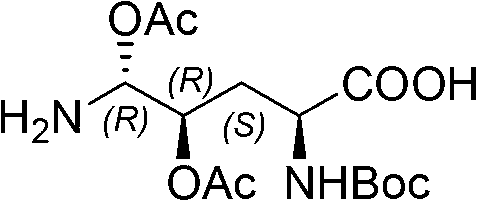
[0043] (1) Synthesis of ortho-dihydroxyl groups by Sharpless asymmetric dihydroxylation reaction. 70g (217mmol) K 3 Fe(CN) 6 , 29.3g (217mmol) K 2 CO 3 , 53.2 mg (0.14 mmol) K 2 OSo 2 (OH) 4 And 0.56g (0.7mmol) hydroquinidine 1,4-(2,3-naphthyridine) diether ((DHQD) 2 -PHAL) was dissolved in 350ml tert-butanol and 350ml water, and stirred at room temperature for 10 minutes. Add 6.7g (70mmol) ammonium methanesulfonate, and at 0°C, Boc-4-ene-L-ornithine lactam (JournalofOrganometallicChemistry691 (2006), 5487-5496, experimental part 4.3) prepared according to the method of known literature .3, Compound 24) 14.9g (70mmol) was added to the system, and kept at 0°C for 40h. Subsequently, 105 g (833 mmol) of sodium sulfite was added, the reaction was naturally raised to room temperature and stirred for 1 hour, and 700 ml of ethyl acetate was added. The aqueous layer was extracted with ethyl acetate (3 x 200m...
Embodiment 2
[0046] Embodiment 2: the synthesis of Fmoc-P-CTC resin
[0047] Weigh 20.0 g of 2-CTC resin with a substitution degree of 0.7 mmol / g, add it to a solid-phase reaction column, and wash it twice with DMF. After swelling the resin with DMF for 30 minutes, take 24.0g of Fmoc-P-OH and dissolve it in DMF, add 14.6mL of DIPEA in an ice-water bath to activate for 3 minutes, then add it to the above-mentioned reaction column filled with resin, react for 2 hours, add 20mL of anhydrous Methanol blocked for 1 hour. Wash 3 times with DMF and 3 times with DCM, seal with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-P-CTC resin, the detection degree of substitution is 0.56mmol / g.
Embodiment 3
[0048] Example 3: Synthesis of (4S)-N-Fmoc-4-acetoxy-4-(4'-acetoxy)phenyl-L-threonine:
[0049] Dissolve 50g (185mmol) of p-benzyloxybenzaldehyde in 250ml of freshly distilled DCM, add 101.4g (185mmol) of phosphine ylide, and stir at room temperature for 4 hours. The reaction solution is purified by silica gel flash column chromatography to obtain 80.1g of white solid. Dissolve this solid in 300ml of freshly distilled DCM, add a solution of 15g (277mmol) sodium methoxide in 2300ml of anhydrous methanol, stir and react at room temperature for 30 minutes, add 1500ml of 1mol / L hydrochloric acid solution to acidify, spin off the organic solvent, and use the remaining liquid DCM extraction (3 x 600ml). The organic phases were combined, washed twice with saturated brine, dried over anhydrous sodium sulfate for 2 h, and then concentrated under reduced pressure to remove the solvent. The residue and 152.3g (302mmol) 2,3,4-triacetyl-D-methylglucopyranosyl-(N-phenyl)-2,2,2-trifluoroace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com