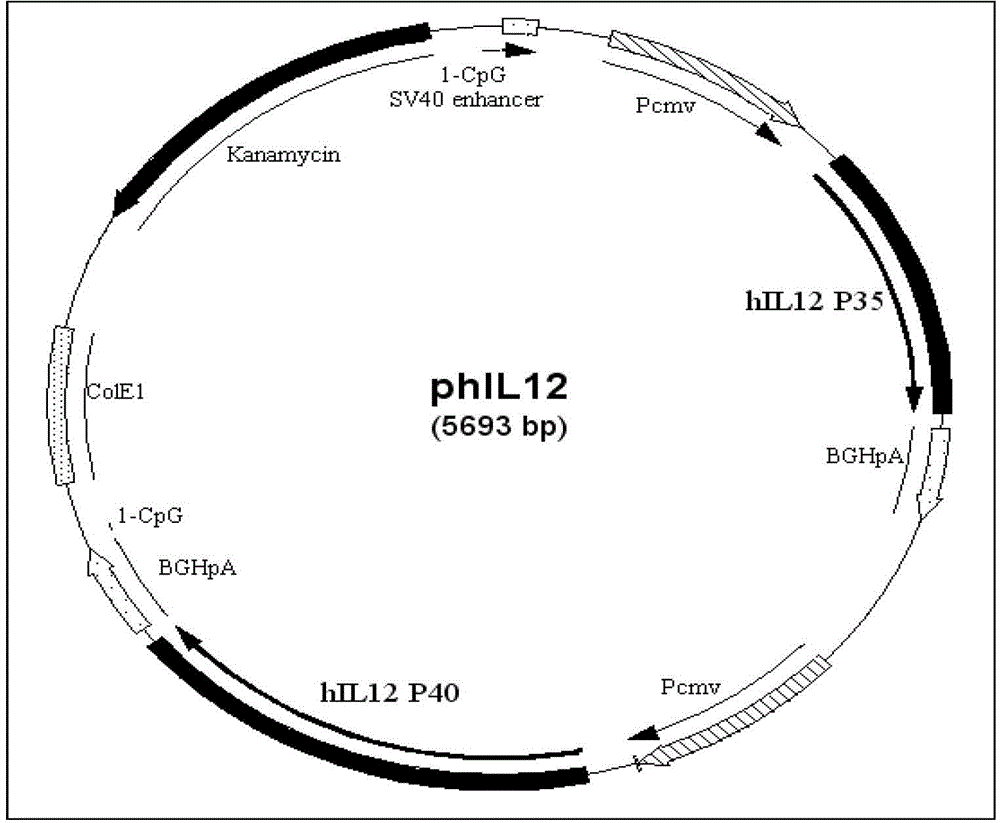
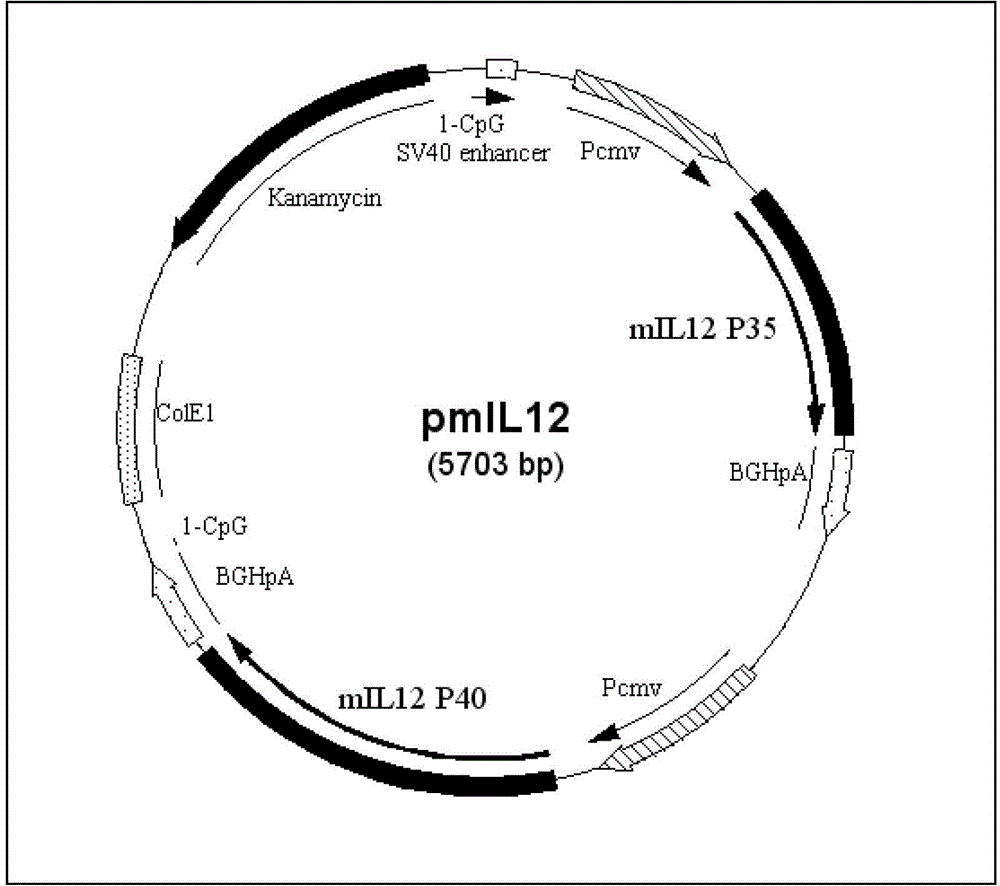
Recombinant plasmid vaccine for treating hepatitis B and composition thereof
A technology of recombinant plasmids and plasmids, which is applied in the field of biomedicine and can solve the problem that the immune effect does not reach the ideal level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1 Construction of recombinant plasmid pRec2.0-preS2S-C (abbreviated as pS2S-C)
[0104] (1) First construct the recombinant plasmid vector backbone pOE-EKS, whose nucleotide sequence is the nucleotide sequence indicated by Seq No.1:
[0105] Using pDRVISV1.0 as a template, Primer1 and Primer2 as primers, among which primer 2 is a primer phosphorylated at the 5' end, amplified to obtain a replicon region (Ori) with a size of 748bp, and introducing EcoRI, KpnI and SwaI restriction sites at the same time point;
[0106] Primer1: 5'-GGAATTCGGGGTACCATTTAAATTTGAACGTTCGCAAtATGTGAGCAAAAGGCCAGC-3'
[0107] Primer2: 5'-CGGCGCGCGCCGAAAACGACGATTGCGAACGTTCAACCCGTAGAAAAAGATCAAAGG-3'
[0108] Using pDRVISV1.0 as a template, Primer3 and Primer4 as primers, among which primer 3 is a primer phosphorylated at the 5' end, amplified to obtain a Kanna resistance marker gene (Kan) with a size of 1056 bp, and introducing an EcoRI restriction site ;
[0109] Primer3: 5'-tcgtcgttttc...
Embodiment 2
[0129] The Core expression cassette was excised from the plasmid pcDNA3.1-C by BglII+PvuII double digestion, and cloned into pRec2.0-preS2S which was digested by BglII+EcoRV to construct the recombinant plasmid pRec2.0-PreS2S-C. Example 2 Construction of recombinant plasmid pRec2.0-C-preS2S (pC-S2S)
[0130] Using the pRec2.0-PreS2S constructed in Example 1 as a template, using Primer11 and Primer12 as primers, amplify the PreS2S gene with a size of 873bp, and pass HindIII+XbaI double digestion and HindIII+XbaI double digestion vector pcDNA3 .1 connect, construct and obtain pcDNA3.1-PreS2S;
[0131] Primer11:5'-CCCAAGCTTGCCGCCACCATGCAGTGGAACTC-3'
[0132] Primer12:5'-GCTCTAGAATCAGATGTAAACCCAC-3'
[0133] The PreS2S expression cassette was excised from the plasmid pcDNA3.1-PreS2S by BglII+PvuII double enzyme digestion, and cloned into pRec2.0-C which was digested by BglII+EcoRV double enzymes, and the recombinant plasmid pRec2.0-C-PreS2S (pC -S2S).
Embodiment 3
[0134] Example 3 Construction of recombinant adjuvant plasmid pmIL12
[0135] (1) The synthetic gene mP35 is connected with the carrier pcDNA3.1 of HindIII+XbaI double digestion by HindIII+XbaI double digestion, and the recombinant plasmid pcDNA3.1-mP35 is constructed and obtained;
[0136] (2) The mP35 gene expression cassette was cut out from the plasmid pcDNA3.1-mP35 by BglII+PvuII double digestion, and cloned into the plasmid pRec2.0-PreS2S which was digested by BglII+EcoRV to obtain the recombinant plasmid pRec2.0-PreS2S -mP35;
[0137] (3) Ligate the synthetic gene mP40 with the HindIII+XbaI double-digested vector pcDNA3.1 through HindIII+XbaI double-digestion to construct and obtain the recombinant plasmid pcDNA3.1-mP40;
[0138] (4) The mP40 gene expression cassette was cut out by SalI+PvuII double enzymes, and cloned into the plasmid pRec2.0-PreS2S-mP35 cut by SalI+SwaI double enzymes to obtain the recombinant plasmid pRec2.0-PreS2S-mIL12;
[0139] (5) Digest the pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com