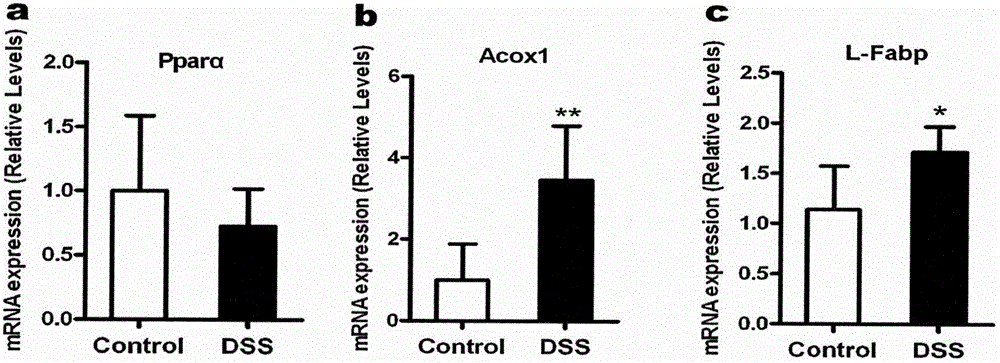
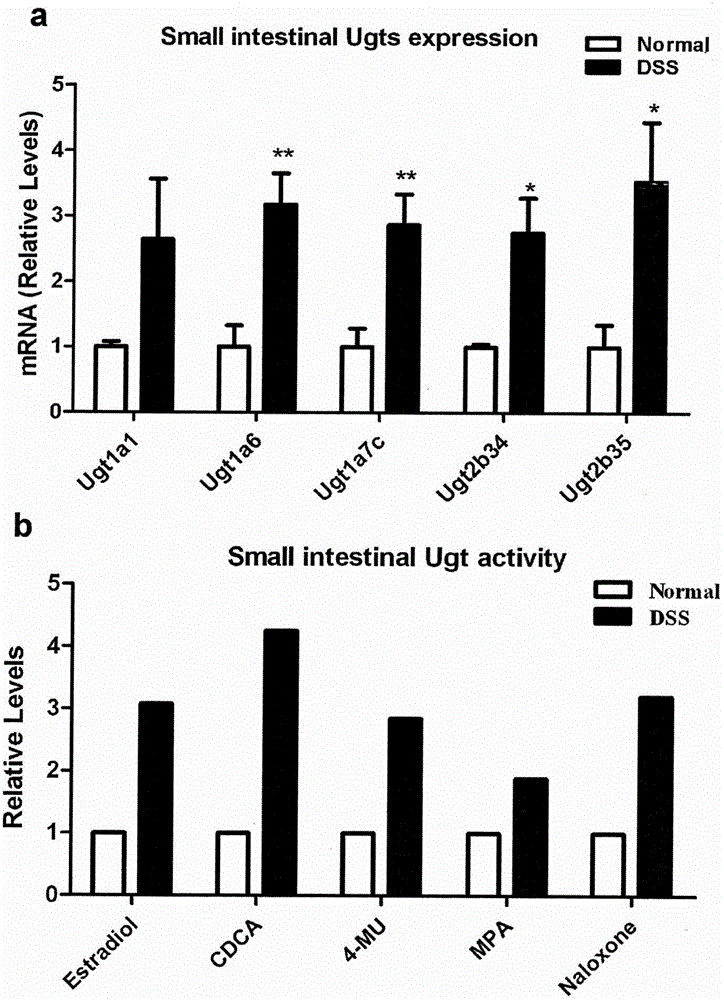
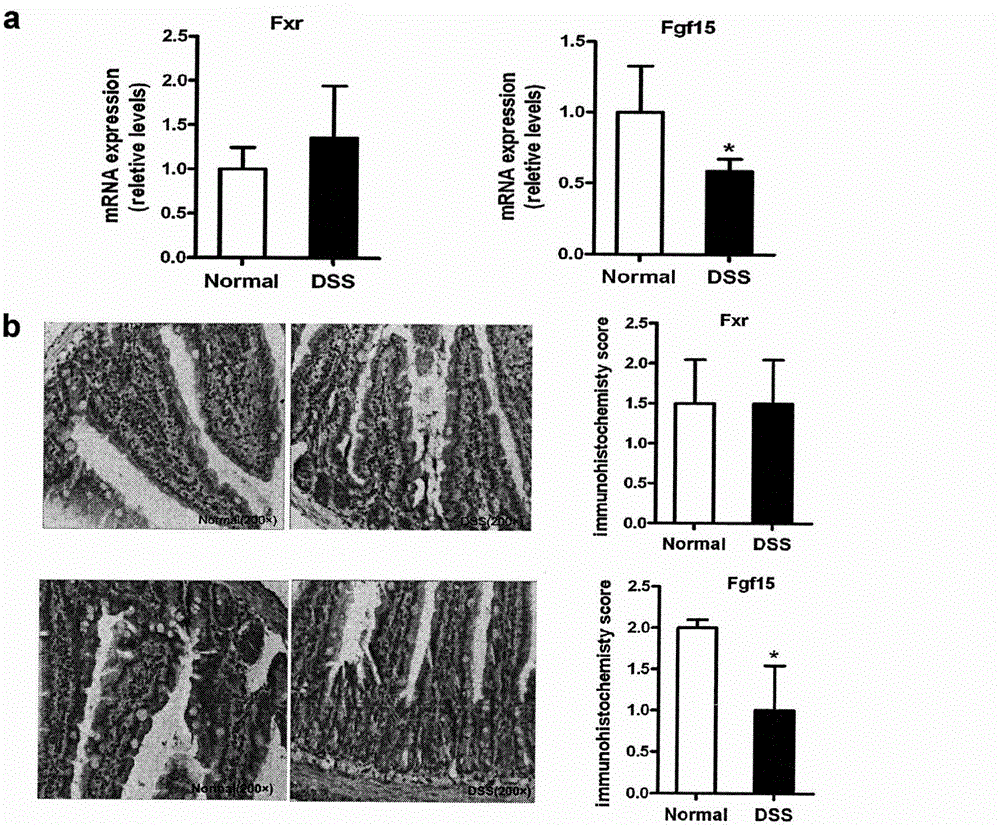
Application of PPAR alpha-UGT (peroxisome proliferator activated receptor alpha-uridine diphosphate glucuronosyl transferase) pathway inhibitor in treating inflammatory bowel diseases
An inflammatory bowel disease and inhibitor technology, applied in the field of new drug research and development, can solve the problems of impairing the patient's immune defense ability, increasing the risk of malignant lesions, and reducing the patient's ability to resist infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1. Replication of DSS-induced chronic colonic inflammation mouse model
[0012] Experimental materials: 20 g of SPF male C57BL / 6 mice (8 weeks old), provided by the Experimental Animal Center of the Academy of Military Medical Sciences;
[0013] Dextran sulfate (DSS, 36000-50000) was purchased from MP Company.
[0014] experimental method:
[0015] 1. The experimental mice need to adapt to the standardized animal room environment for at least 7 days, give standard animal feed, drink autoclaved water, and change the litter in time (2-3 times a week).
[0016] 2. Prepare 2.5% DSS solution: Use autoclaved water to prepare 2.5% DSS solution according to the weight-to-volume ratio. The prepared DSS solution can be stored in a refrigerator at 4°C for one week.
[0017] 3. After the start of the experiment, according to the daily drinking volume of each mouse 5ml, pour the DSS solution of the 2-day drinking water into the mouse water bottle each time. If there is any...
Embodiment 2
[0021] Example 2.qRT-PCR gene expression detection
[0022] Experimental materials: Trizol (Takara, Japan), reverse transcription reagents (Takara, Japan), SYBR Green I PCR kit (Takara, Japan), Bio-Rad CFX series fluorescence quantitation instrument (Biosystems, Bedford, MA).
[0023] Experimental method: Total RNA was extracted with Trizol (Takara, Japan), and the RNA concentration and purity were determined. Reverse transcription was performed using a reverse transcription reagent (Takara, Japan). And with quantitative PCR instrument: Thermal Cycler Dice TM Experiments were performed on Real Time System (Takara, Japan). Primer sequences are shown in Table 1. SYBR Green I PCR kit (Takara, Japan) was used for gene expression research. A three-step method was adopted, the reaction system was 20 μL, the annealing temperature was 60°C, and each sample was repeated three times, and the data processing was performed by ΔΔC t Law.
[0024] Table 1 qRT-PCR primer sequences
...
Embodiment 3
[0027] Embodiment 3.Western Blot detection
[0028] Experimental materials: Bio-Rad electrophoresis instrument; Bio-Rad gel imager. FXR, FGF15, Cyp7a1 antibodies and corresponding secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). GAPDH antibody was purchased from SunShine Biotechnology (Nanjing, China). Cell lysate: 10mM Tris-HCl (pH7.5), 1mM EGTA, 1mM MgCl2, 1mM β-ME, 1% glycerine, protease inhibitor (containing 1mM DTT and 2mM PMSF).
[0029] experimental method:
[0030] 1. Take an appropriate amount (80-100mg) of fresh tissue samples or properly preserved tissue samples, add 1ml of total protein extraction reagent (or nucleoprotein extraction reagent) containing protease inhibitors, and extract total protein (or nuclear protein extraction reagent) after homogenization protein).
[0031] 2. Protein quantification: Follow the operation instructions of the BCA protein quantification kit to determine the concentration of the sample.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com