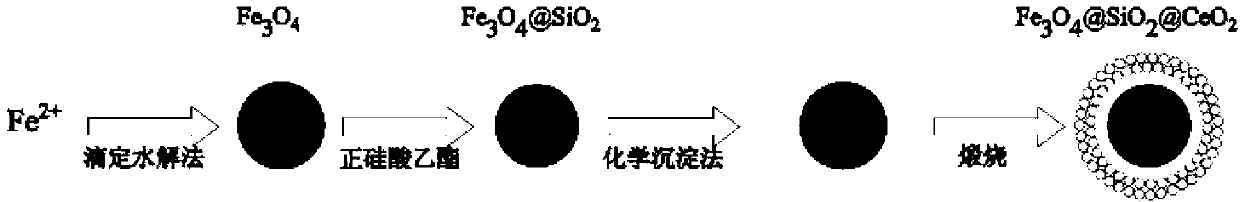
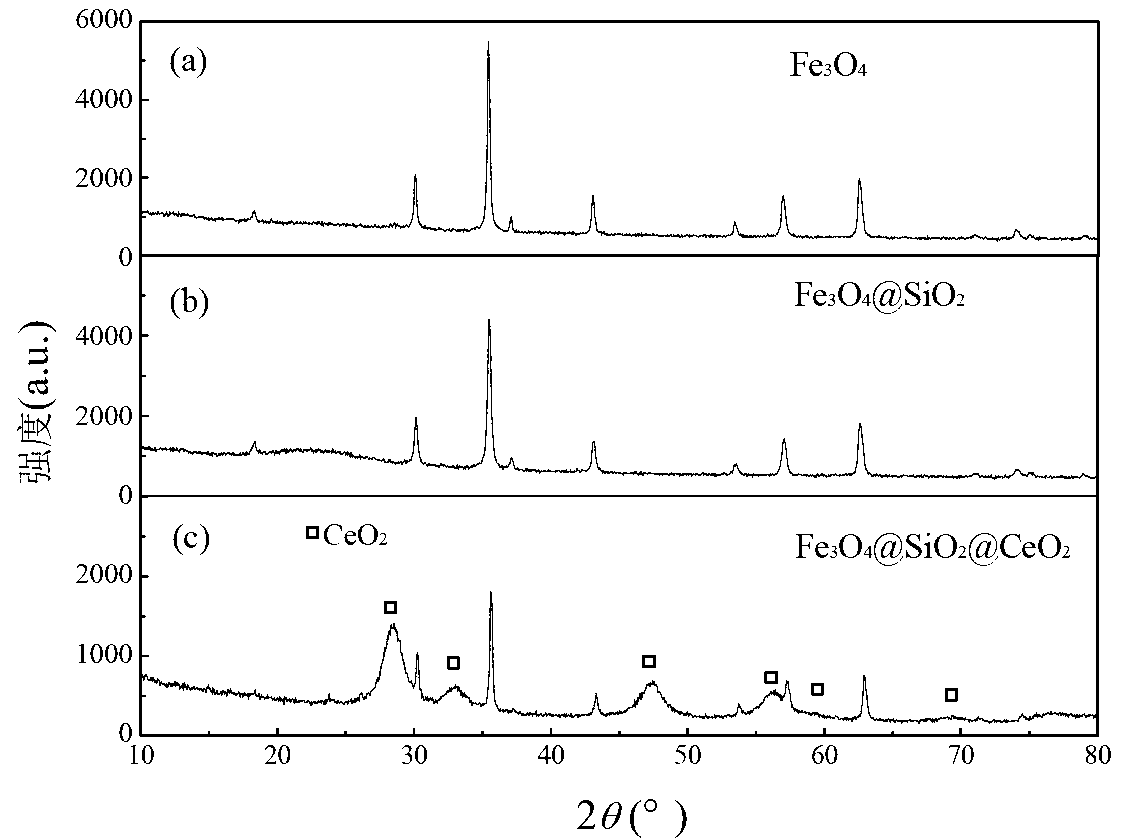
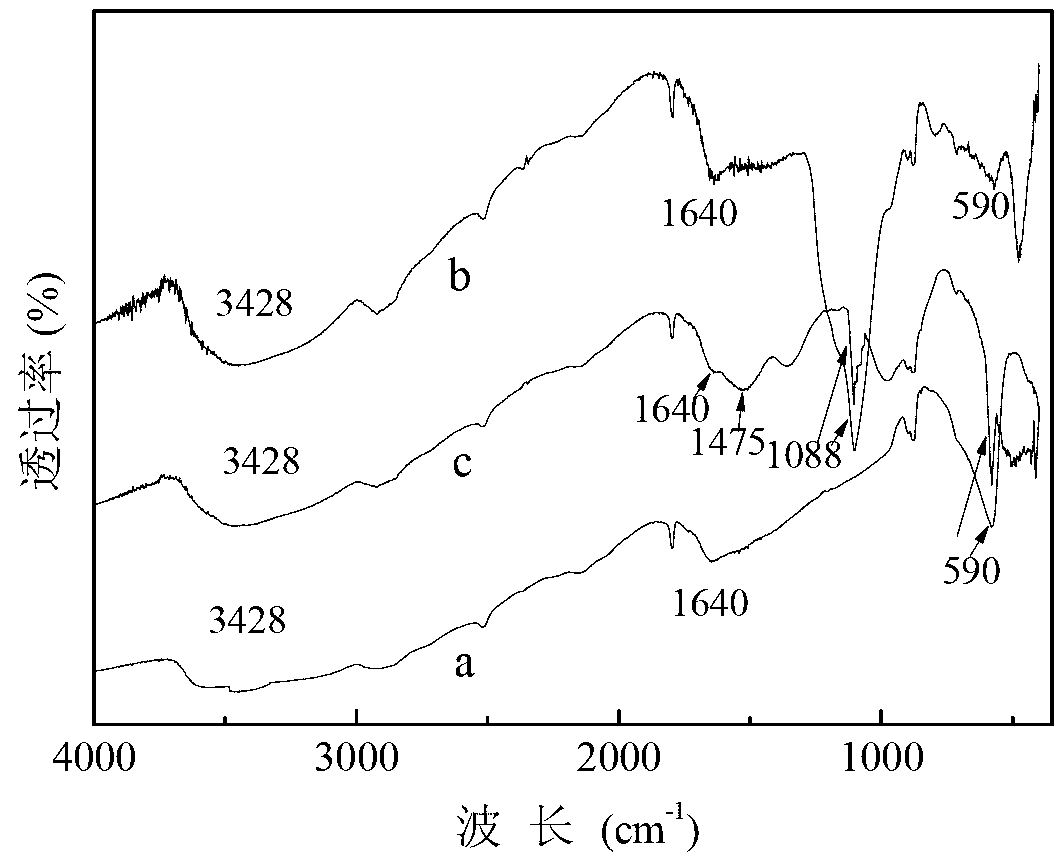
Magnetic nano cerium dioxide ozone catalyst, preparation method and application
A technology of cerium dioxide and magnetic nanometers, which is applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of catalyst difficulty and separation, and achieve easy recycling The effect of reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Nano Fe 3 o 4 preparation of
[0038] The titration hydrolysis method was used for the preparation of nano-ferric oxide. First, the deionized water is aerated with high-purity nitrogen for 1 hour to remove oxygen in the deionized water, and the deionized water for preparing solutions in subsequent steps is prepared with the deoxygenated deionized water. Then KNO 3 (10.12g) and KOH (5.62g) were dissolved in 400ml of deoxygenated deionized water, and 100ml of 0.2mol / L FeSO 4 solution, accompanied by nitrogen protection. After 4 hours of stirring, a black precipitate was formed in the solution, and the black precipitate was separated by magnetism and washed 3 times with ethanol and 3 times with deionized water, and dried in a vacuum oven at 70°C to obtain black nano-Fe 3 o 4 particles.
[0039] (2) Nano Fe 3 o 4 SiO 2 preparation of
[0040] Preparation of nano-Fe 3 o 4 SiO 2 Particles, using the principle of tetraethyl orthosilicate (TEOS) hydrolysis, in...
Embodiment 2
[0052] Preparation of target pollutant aspirin solution: Dissolve a certain amount of aspirin in water to prepare an aspirin solution with a concentration of 1000 mg / L.
[0053] In the aspirin solution of 1000mg / L of 1.5L above-mentioned preparations, add 1.5g of magnetic cerium dioxide ozone catalyst prepared by the method of embodiment 1, after mixing evenly, add in the internal circulation ozone catalytic reactor to react and aerate for 120min, use high-efficiency liquid Phase chromatography and TOC detector detect the amount of aspirin and TOC in the solution during the reaction. Ozone, Fe 3 o 4 and Fe 3 o 4 SiO 2 As a comparative experiment.
[0054] The change curve of aspirin content with reaction time is as follows Figure 6 As shown in a, the removal rate of aspirin with the reaction time can be obtained correspondingly. After 60 minutes of reaction, the Fe 3 o 4 SiO 2 CeO 2 As a catalyst, the removal rate of aspirin was 81.0%, while Fe 3 o 4 and Fe 3 o 4 ...
Embodiment 3
[0056] With reference to the method preparation concentration among the embodiment 1, be the aspirin solution of 1000mg / L. In the aspirin solution of 1000mg / L of 1.5L above-mentioned preparations, add 1.5g of magnetic cerium dioxide ozone catalyst prepared by the method of embodiment 1, after mixing evenly, add in the internal circulation ozone catalytic reactor to react and aerate for 120min, use high-efficiency liquid Phase chromatography and TOC detector detect the amount of aspirin and TOC in the solution during the reaction. After the reaction, the catalyst was recovered by magnetic separation, and the experiment was continued for 3 times according to the above method. Compare the effect of catalyst reuse.
[0057] Experimental results such as Figure 7 Shown, are successively the change curve of aspirin content with reaction time in 3 experiments. After three consecutive reactions, the catalytic activity of the magnetic ceria ozone catalyst did not decrease significan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com