Application of terpene compound
A technology of terpenoids and drugs, applied in the field of natural medicinal chemistry, can solve the problems of unclear antifungal active ingredients and achieve the effect of preventing and/or treating fungal infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Isolation and preparation of formula I compound
[0023] Dried Kochia scoparia medicinal material, pulverized, subjected to CO 2 Supercritical extraction to remove grease, the dregs were extracted three times with 80% ethanol reflux, the extract was concentrated, the obtained extract was mixed with diatomaceous earth (1:1), and petroleum ether, chloroform, ethyl acetate, methanol were eluted in sequence, and the obtained The methanol eluted part was then extracted with water and saturated n-butanol to obtain the n-butanol part. The n-butanol fraction was prepared through repeated silica gel column chromatography, gel Sephadex LH-20 chromatography, and medium-low pressure reversed-phase silica gel liquid phase to obtain the compound of formula I.
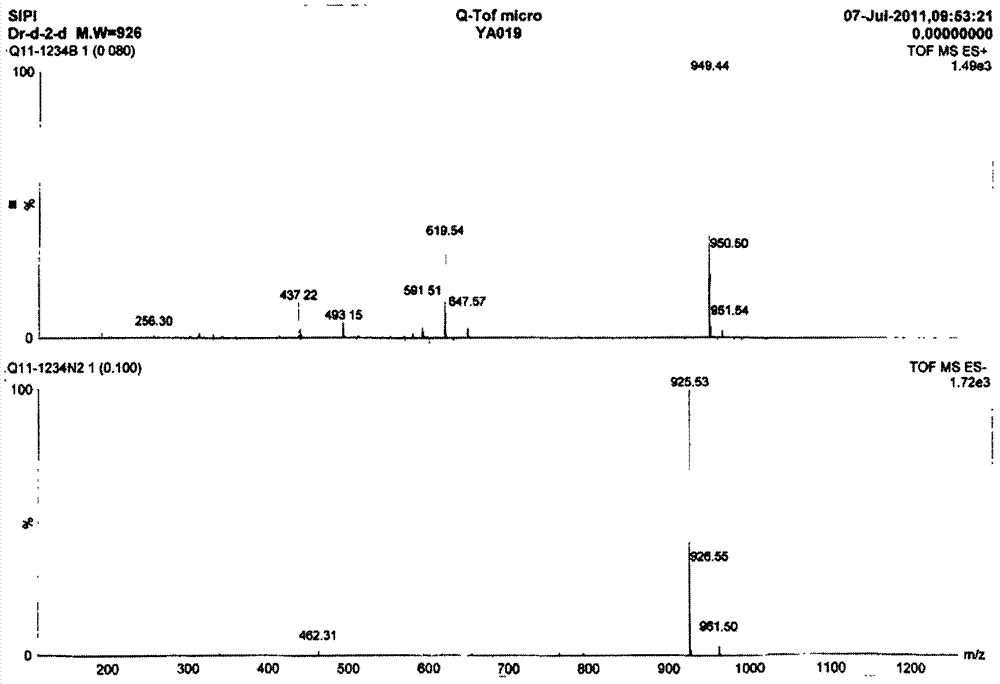
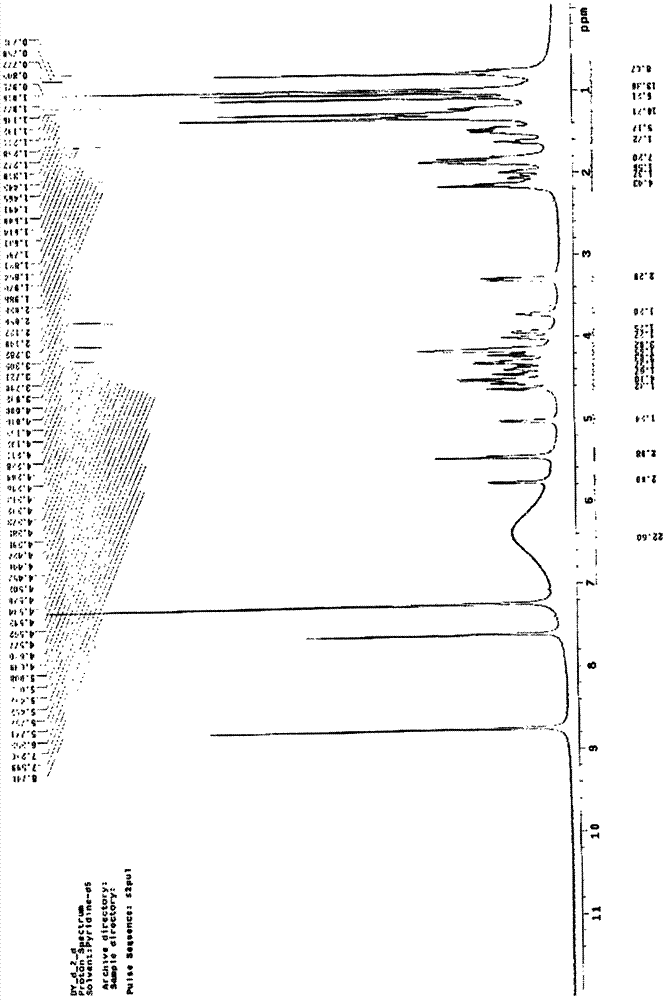
[0024] Formula I compound structure is by ESI-MS, 1 H-NMR and 13 C-NMR identification. ESI-MS measuring instrument: MSD-Trap-XCT, Q-Tof micro (ESI-MS) type mass spectrometer (Agilent Company), 1 H-NMR measur...
Embodiment 2
[0029] Embodiment 2: the antifungal activity determination of formula I compound
[0030] The compound obtained in Example 1 was tested for its antibacterial activity against Candida albicans by the paper sensitive sheet method.
[0031] Preparation of the test solution: take the compound of formula 1 and dissolve it in methanol to prepare 5mg·mL -1 solution, set aside.
[0032] Preparation of bacterial suspension: Take an appropriate amount of 0.85% sterile saline and add it to the activated Candida albicans slant test tube, wash the surface bacteria with an inoculation spatula, and pour it into a sterile small triangular flask with glass beads , shattered by shaking, and then diluted the bacterial solution with normal saline to a bacterial concentration of 10 9 / ml, stored in a refrigerator at 4°C until use.
[0033] Take 1.5ml of Candida albicans solution and place it in 100ml of yeast extract peptone glucose agar medium cooled to 48°C-50°C after melting, shake it up qui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com